FDA Gets Slapped and Removes Tweet: "You are Not a Horse. You are Not a Cow, Seriously, y'all. Stop it." And Leaves Up a Letter to Stakeholders Advising Humans Not to Take Animal Ivermectin
People Take Horse Ivermectin Anyway
A lawsuit fighting back at the FDA’s misinformation on ivermectin now forced the FDA, via a mandate handed down on March 21, 2024, to remove this tweet within twenty one days.
The FDA posts had advised people to stop using human ivermectin as a COVID treatment, and the court found that the FDA is not allowed to give medical advice.
The August 2021 tweet was among other posts on Linkedin, Instagram, and Facebook, who were censoring medical doctors who promoted ivermectin as a simple therapy to both prevent and treat Covid symptoms. Note that the posts cited medical advice NOT to take ANY FORM of ivermectin, and did not specify human pills or capsules vs. horse paste.
While the doctors who brought the lawsuit hailed it as a significant win, ironically, the FDA maintains that they didn’t do anything wrong and the removal is not an admission of wrongdoing.
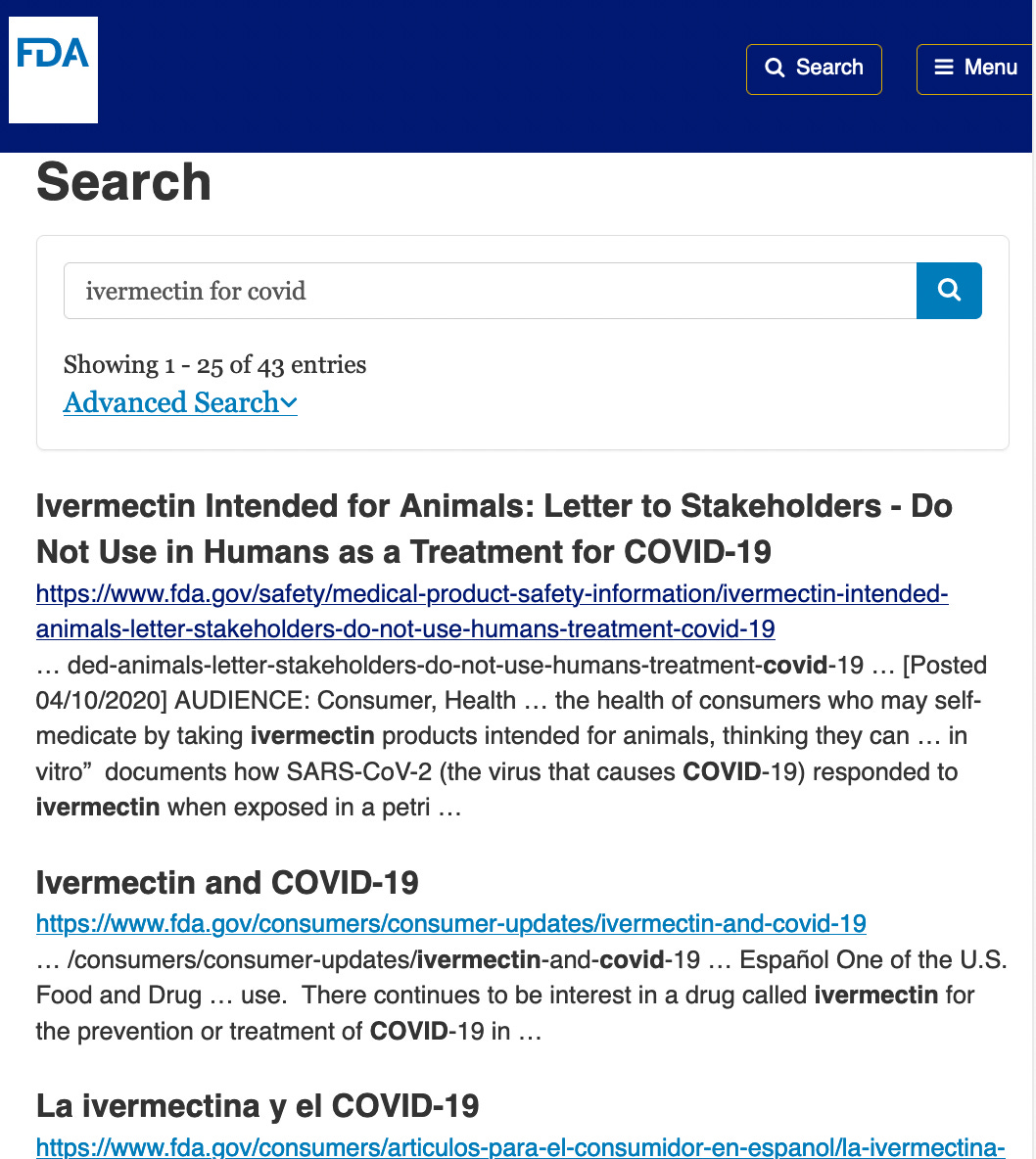
The FDA had stated that ivermectin was not effective against COVID-19, publishing this first site on the use of ivermectin for COVID. Today, a SwissCows.com search for “FDA and ivermectin for Covid” reveals a couple of previews of hits:
The first site, Why You Should Not Use Ivermectin to Treat or PRevent COVID-19 now reveals today:
The second hit shows the following warning letter given to www.ivermectin4covid.com:
The site reads:
Product:
Drugs
Recipient:
www.ivermectin4covid.com
United States
Issuing Office:
Center for Drug Evaluation and Research | CDER
United States
FROM: The United States Food and Drug Administration
RE: Notice of Unlawful Sale of Unapproved and Misbranded Drugs to United States Consumers Over the Internet
DATE: March 16, 2023
WARNING LETTER
This is to advise you that the United States (U.S.) Food and Drug Administration (FDA) recently reviewed your website at the Internet address www.ivermectin4covid.com and has observed that your website introduces into interstate commerce misbranded and unapproved new drugs in violation of sections 301(a), 301(d), and 505(a) of the Federal Food, Drug, and Cosmetic Act (FD&C Act) [21 U.S.C. §§ 331(a), 331(d), and 355(a)].
There is currently a global outbreak of respiratory disease caused by a novel coronavirus that has been named “severe acute respiratory syndrome coronavirus 2” (SARS-CoV-2). The disease caused by the virus has been named “Coronavirus Disease 2019” (COVID-19). On January 31, 2020, the Department of Health and Human Services (HHS) issued a declaration of a public health emergency related to COVID-19 and mobilized the Operating Divisions of HHS.1 In addition, on March 13, 2020, there was a Presidential declaration of a national emergency in response to COVID-19.2 Therefore, FDA is taking urgent measures to protect consumers from certain products that, without approval or authorization by FDA, claim to mitigate, prevent, treat, diagnose, or cure COVID-19 in people. As described below, FDA has observed that your website offers drug products for sale in the U.S. that are intended to mitigate, prevent, treat, diagnose, or cure COVID-19.
There are inherent risks to consumers who purchase unapproved new drugs and misbranded drugs. Unapproved new drugs do not carry the same assurances of safety and effectiveness as those drugs subject to FDA oversight. Drugs that have circumvented regulatory safeguards may be contaminated, counterfeit, contain varying amounts of active ingredients, or contain different ingredients altogether. We request that you cease the sale of any unapproved and misbranded products, whether for the mitigation, prevention, treatment, diagnosis, or cure of COVID‐19, or any other disease for which the drugs you are selling are not approved by FDA for distribution in the U.S.
Unapproved New Drugs:
Certain products offered for sale by www.ivermectin4covid.com are drugs within the meaning of section 201(g) of the FD&C Act [21 U.S.C. § 321(g)] because they are intended for use in the diagnosis, cure, mitigation, treatment, or prevention of disease and/or because they are intended to affect the structure or function of the body. These drugs are also new drugs as defined by section 201(p) of the FD&C Act [21 U.S.C. § 321(p)], because they are not generally recognized as safe and effective for their labeled uses. With certain exceptions not applicable here, new drugs may not be legally introduced or delivered for introduction into interstate commerce without prior approval from FDA, as described in section 505(a) of the FD&C Act [21 U.S.C. § 355(a)]. No approved applications pursuant to section 505 of the FD&C Act are in effect for these products. Accordingly, their introduction or delivery for introduction into interstate commerce violates sections 301(d) [21 U.S.C. § 331(d)] and 505(a) of the FD&C Act.
For example, www.ivermectin4covid.com offers ivermectin marketed as “Iverheal 12mg” manufactured by Healing Pharma. Your website states, “Ivermectin (Iverheal 12) is an antiparasitic, and also an antiviral drug manufactured by Healing Pharma. It is used to kill the parasites in the body. It is also useful in Covid 19 care.” While there are FDA-approved versions of ivermectin on the market in the U.S., there are no approved drug applications pursuant to section 505 of the FD&C Act in effect for the “Iverheal 12mg ” manufactured by Healing Pharma and offered by www.ivermectin4covid.com. FDA-approved ivermectin tablets are approved for the treatment of intestinal (i.e., nondisseminated) strongyloidiasis due to the nematode parasite Strongyloides stercoralis and onchocerciasis due to the nematode parasite Onchocerca volvulus, and are only available by prescription. In addition, ivermectin has not been approved by FDA for use in the prevention, diagnosis, treatment, mitigation, or cure of COVID-19.
Misbranded Drugs:
A drug is misbranded under section 502(f)(1) of the FD&C Act [21 U.S.C. § 352(f)(1)] if its labeling fails to bear adequate directions for use. “Adequate directions for use” means directions under which a layperson can use a drug safely and for the purposes for which it is intended (see 21 CFR 201.5). Prescription drugs, as defined in section 503(b)(1) of the FD&C Act [21 U.S.C. § 353(b)(1)] include those that, because of their toxicity or other potentiality for harmful effect, or the method of their use, or the collateral measures necessary for their use, are not safe for use except under supervision of a practitioner licensed by law to administer them. Prescription drugs, as defined in section 503(b)(1) of the FD&C Act, can be used safely only at the direction, and under the supervision, of a licensed practitioner.
Because the aforementioned drugs are prescription drugs intended for conditions that are not amenable to self-diagnosis and treatment by a layperson, adequate directions cannot be written such that a layperson can use the products safely for the intended uses. Consequently, the labeling for these drugs fails to bear adequate directions for use, causing them to be misbranded under section 502(f)(1) of the FD&C Act. In addition, because the drugs are not approved in the U.S., they are also not exempt under 21 CFR 201.115(a) from the requirements of section 502(f)(1) of the FD&C Act. By offering these drugs for sale to U.S. consumers, www.ivermectin4covid.com is causing the introduction of misbranded drugs into interstate commerce in violation of section 301(a) of the FD&C Act [21 U.S.C. § 331(a)].
FDA is sending this Warning Letter to www.ivermectin4covid.com because of the inherent risks to consumers who purchase misbranded and unapproved new drugs. This letter is not intended to identify all the ways in which your products or operations might be in violation of the law. It is your responsibility to ensure that all products you offer for sale are in compliance with the FD&C Act and its implementing regulations. You should take prompt action to address any violations of the FD&C Act (which may include the offer for sale of similarly misbranded and/or unapproved new drugs other than the drugs noted above). We advise you to review your website, product labels, and other labeling and promotional materials to ensure that you are not misleadingly representing your products as safe and effective for a use for which they have not been approved by FDA and that you are not distributing misbranded products in violation of the FD&C Act.
Within 48 hours, please send an email to FDAInternetPharmacyTaskForce-CDER@fda.hhs.gov and COVID-19-Task-Force-CDER@fda.hhs.gov describing the specific steps you have taken to address any violations and to prevent their recurrence. Include an explanation of each step being taken to remedy and prevent the recurrence of any violations, as well as copies of related documentation. Failure to adequately address this matter may result in legal action, including, without limitation, seizure and injunction, without further notice. If you cannot complete corrective action within 48 hours, state the reason for the delay and the time within which you will complete the corrections. This letter notifies you of our concerns and provides you with an opportunity to address them. If you believe that your products are not in violation of the FD&C Act, include your reasoning and any supporting information for our consideration within 48 hours.
If you are not located in the U.S., please note that products that appear to be misbranded or unapproved new drugs may be detained or refused admission. We may advise the appropriate regulatory officials in the country from which you operate that your products referenced above appear to be unapproved and misbranded products that cannot be legally sold to consumers in the U.S.
Please direct any inquiries to FDA at FDAInternetPharmacyTaskForce-CDER@fda.hhs.gov and COVID-19-Task-Force-CDER@fda.hhs.gov.
Sincerely,
/S/
S. Leigh Verbois, Ph.D.
Director, Office of Drug Security, Integrity, and Response
Office of Compliance
Center for Drug Evaluation and Research__________________________
1 Secretary of Health and Human Services, Determination that a Public Health Emergency Exists (originally issued Jan. 31, 2020, and subsequently renewed), available at https://www.phe.gov/emergency/news/healthactions/phe/Pages/default.aspx.
2 Proclamation on Declaring a National Emergency Concerning the Novel Coronavirus Disease (COVID-19) Outbreak. Mar. 13, 2020, 85 FR 15337, available at https://www.federalregister.gov/documents/2020/03/18/2020-05794/declaring-a-national-emergency-concerning-the-novel-coronavirus-disease-covid-19-outbreak.
The FDA Website Continues to Advise Humans Not To Take Animal Ivermectin
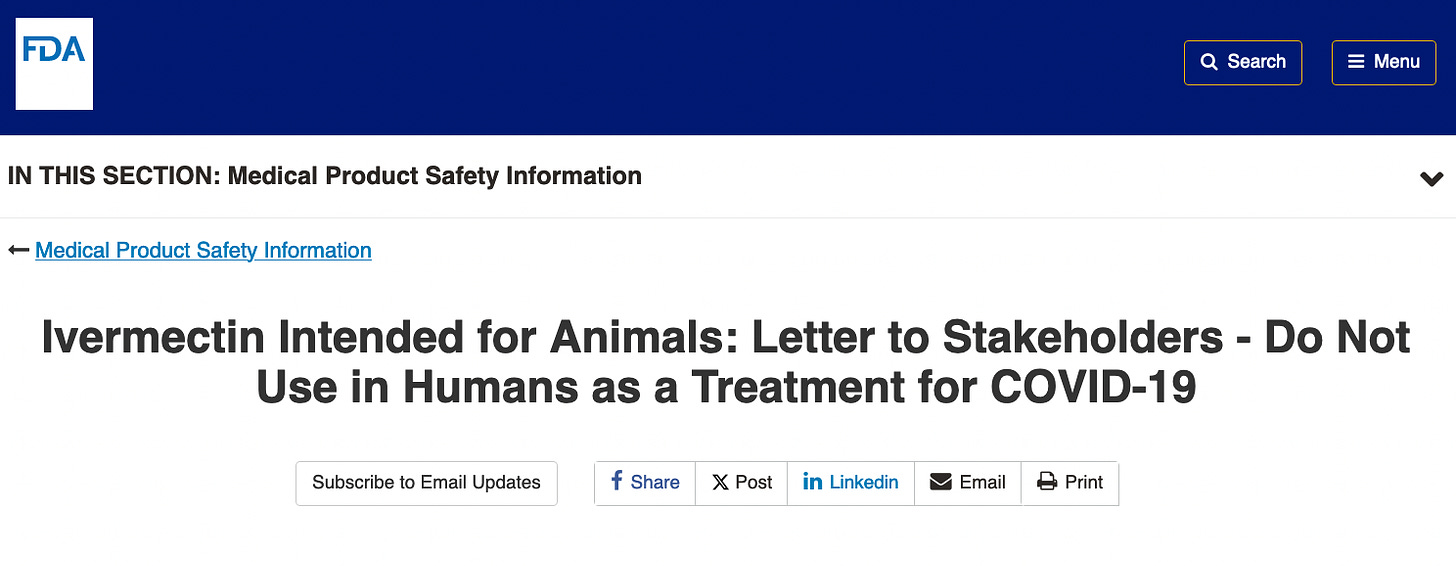
A search of the FDA.gov website for “ivermectin for Covid” shows an article in a letter to stakeholders entitled, “Do Not Use in Humans as a Treatment for COVID-19”.
Today, the first article goes here: https://www.fda.gov/safety/medical-product-safety-information/ivermectin-intended-animals-letter-stakeholders-do-not-use-humans-treatment-covid-19 and shows this April 4, 2010 article advising people not to take animal ivermectin, which is different than telling people not to take human ivermectin:
The “Recommendation” now confines itself to ivermectin for animals, and cites not to take human ivermectin unless prescribed. So you can tell they are being more careful, and have not edited this page since April 10, 2010. I suspect that if they had left it at this and not raised the bar to call you a horse or a cow, there never would have been a lawsuit.
RECOMMENDATION:
People should never take animal drugs, as the FDA has only evaluated their safety and effectiveness in the particular animal species for which they are labeled. These animal drugs can cause serious harm in people.
People should not take any form of ivermectin unless it has been prescribed by a licensed health care provider and is obtained through a legitimate source.
Ivermectin is an important part of a parasite control program for certain species and should only be given to animals for approved uses or as prescribed by a veterinarian in compliance with the requirements for extra-label drug use.
If you are having difficulty locating a particular ivermectin product for your animal(s), FDA recommends that you consult with your veterinarian.
Please help us protect public health by alerting FDA of anyone claiming to have a product to prevent or cure COVID-19 and to help safeguard human and animal health by reporting any of these products to FDA-COVID-19-Fraudulent-Products@fda.hhs.gov or 1-888-InfoFDA (1-888-463-6332).
[04/10/2020 – Letter to Stakeholders - FDA]
Taking Ivermectin Horse Paste
Of course, many people could not get their hands on human ivermectin during COVID. In August 2021, the New York Times reported there were over 88,000 legitimate prescriptions of ivermectin per week, with drug shortages.
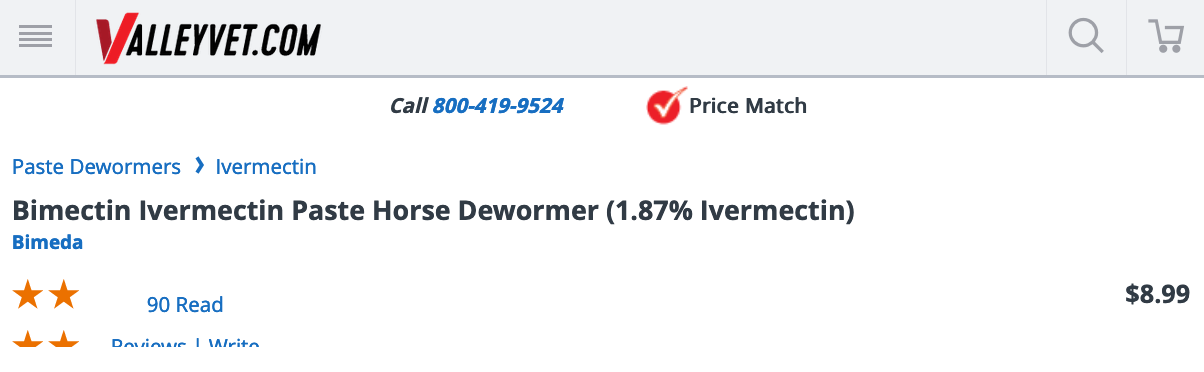
Many could not afford a telemedicine appointment cost plus that of compounded or tablet form ivermectin. Instead, they just went to their local Tractor Supply and bought ivermectin paste off the shelf or from a variety of suppliersonline, without a prescription. Here’s one for $8.99:
So now, the only thing that the FDA has to concentrate on is telling people not to take horse paste ivermectin.
… but people have been doing it, and are going to keep doing it anyway.
Keep reading with a 7-day free trial
Subscribe to The Rebel Patient™ to keep reading this post and get 7 days of free access to the full post archives.