Orabloc™ (Articaine plus Epinephrine) Local Anesthetic Under the Microscope: Unidentified Objects Seen by Will at Micronaut.Substack.com
SEEKING: Specialists from All Fields to Pitch In and Generate Object Identity and Treatment
Image Source: https://www.rxlist.com/orabloc-drug.htm
Will on Micronaut.Substack.com recently showed the dental local anesthetic Orabloc™ under the microscope.
Will’s images show it contains bubbles that churn in specific ways, and also show unidentified objects that self-assemble before one’s very eyes.
It has people scared to let their dentist put numbing medication on their gums before any tooth surgery.
We are grateful that Will on Micronaut.Substack.com recently published this article, seen HERE and below. I became aware of it just days ago. See the article images above and below the link to Will’s article, below:
Dental local anesthetic Orabloc(TM) under a microscope (from above article):
Thank you for posting such incredible microscope videos, Will.
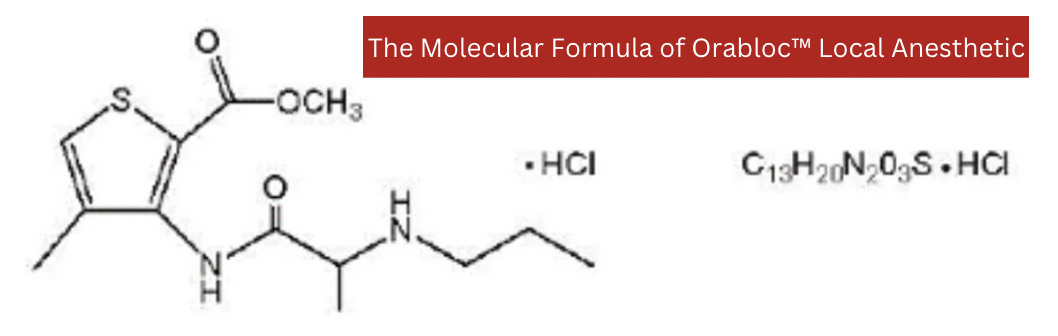
The Chemical Formula of Orabloc™
According to RxList.com, seen HERE, these are the contents and structural formulations of Orabloc™:
Generic Name: articaine hcl and epinephrine injection
Brand Name: Orabloc
Drug Class: Local Anesthetics, Amides, Local Anesthetics, Dental
Orabloc (articaine HCl and epinephrine) is a combination an amide local anesthetic and a vasoconstrictor indicated for local, infiltrative, or conductive anesthesia in both simple and complex dental procedures in adults and pediatric patients 4 years of age and older.
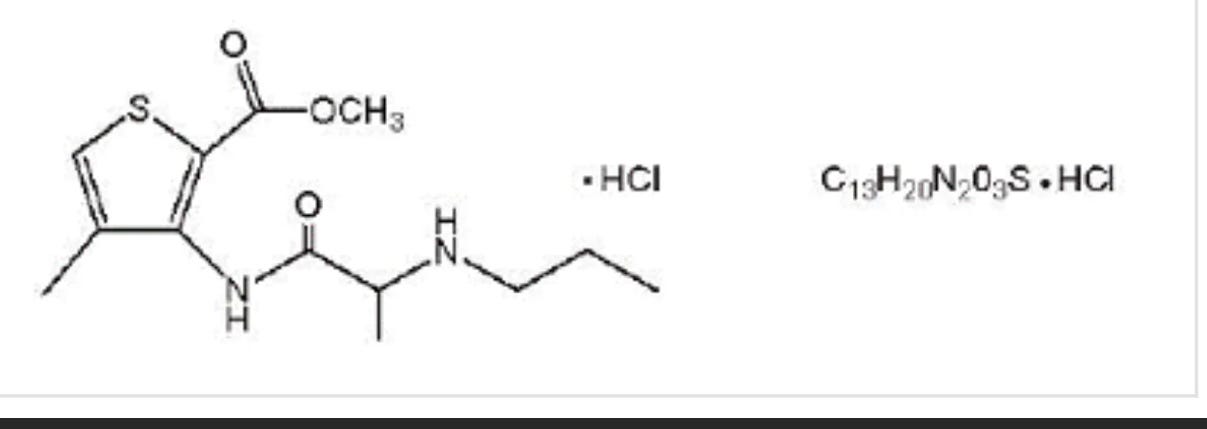
Articaine HCl is an amino amide local anesthetic, chemically designated as 4-methyl-3-[2-(propylamino)- propionamido]- 2-thiophene-carboxylic acid, methyl ester hydrochloride and is a racemic mixture. Articaine HCl has a molecular weight of 320.84 and the following structural formula:
Description for Orabloc
ORABLOC® (articaine hydrochloride and epinephrine injection), for intraoral submucosal infiltration use, is a sterile, aqueous solution that contains articaine HCl 4% (40mg/mL) and epinephrine bitartrate in an epinephrine 1:200,000 or epinephrine 1:100,000 strength.
Articaine
Description for Orabloc
ORABLOC® (articaine hydrochloride and epinephrine injection), for intraoral submucosal infiltration use, is a sterile, aqueous solution that contains articaine HCl 4% (40mg/mL) and epinephrine bitartrate in an epinephrine 1:200,000 or epinephrine 1:100,000 strength
Articaine HCl is an amino amide local anesthetic, chemically designated as 4-methyl-3-[2-(propylamino)- propionamido]- 2-thiophene-carboxylic acid, methyl ester hydrochloride and is a racemic mixture. Articaine HCl has a molecular weight of 320.84 and the following structural formula:
Articaine HCl has a partition coefficient in n-octanol/Soerensen buffer (pH 7.35) of 17 and a pKa of 7.8.
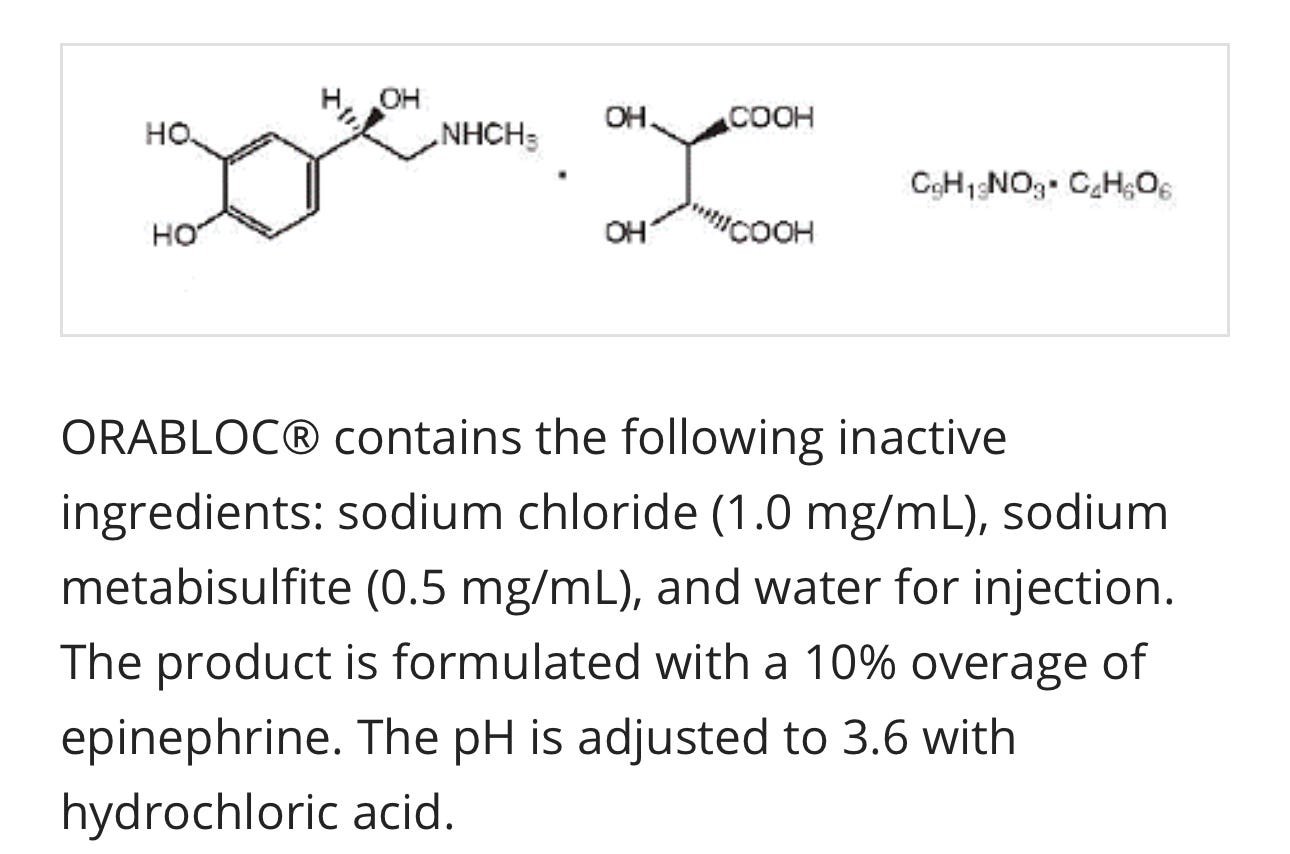
ORABLOC® contains the following inactive ingredients: sodium chloride (1.0 mg/mL), sodium metabisulfite (0.5 mg/mL), and water for injection. The product is formulated with a 10% overage of epinephrine. The pH is adjusted to 3.6 with hydrochloric acid.
…
Epinephrine
Epinephrine bitartrate, (-)-1-(3,4-dihydroxyphenyl)-2-methylamino-ethanol (+) tartrate (1:1) salt, is a vasoconstrictor with a concentration of 1:200,000 or 1:100,000 (expressed as free base). It has a molecular weight of 333.3 and the following structural formula:
The Most Common Use
For most routine dental procedures, ORABLOC containing epinephrine 1:200,000 is preferred. However, when more pronounced hemostasis or improved visualization of the surgical field are required, ORABLOC containing epinephrine 1:100,000 may be used.
This means that in the most common formulation, there is one molecule epinephrine for every 200,000 molecules of articaine. In the double-concentrated version (1:100,000), there is one epinephrine molecule per 100,000 molecules of articaine.
List of Ingredients
Here is the list of ingredients for both concentrations of articaine:epinephrine:
Articaine HCl (a methyl esther hydrochloride) at 40 mg/ml. The chemical name is 4-methyl-3-[2-(propylamino)- propionamido]- 2-thiophene-carboxylic acid, methyl ester hydrochloride.
Epinephrine. Epinephrine bitartrate, -)-1-(3,4-dihydroxyphenyl)-2-methylamino-ethanol (+) tartrate (1:1) salt at 1:200,000 (most common) and 1:100,000, a more concentrated solution. They add an additional 10% ‘overage” of epinephrine.
Sodium chloride (table salt, used also as an IV salt water solution for rehydration), 1 mg/ml.
Hydrochloric acid. Used as a buffer to attain a pH of 3.5, which is quite acidic and explains why it burns upon injection (i.e., because the gum pH is 7.0, which is neutral).
Sodium metabisulfite. 0.5 mg/mL. This small amount of sulphite may cause those with sulpha allergies to have an anaphylactic reaction, which is a medical emergency. According to Wikipedia:
What Drugs, Substances, or Supplements Interact with Orabloc?
Orabloc may interact with monoamine oxidase inhibitors (MAOIs), beta-blockers, tricyclic antidepressants, phenothiazines, abutyrophenones, nitrates/nitrites, other local anesthetics, antineoplastic agents, antibiotics, antimalarials, anticonvulsants, acetaminophen, metoclopramide, quinine, and sulfasalazine. Tell your doctor all medications and supplements you use.
Summary
Orabloc is an amide local anesthetic containing epinephrine and other preservatives, including a sulpha molecule that can cause anaphylaxis with sweating, low blood pressure, hives, lip or tongue swelling, and death by suffocation.
If the epinephrine gets accidentally injected into a small vein or artery, it will cause an immediate, quick elevation in both blood pressure and heart rate - leading the dentist to be alerted to stop injecting.
Future Studies
First, Will’s experiments need to be verified. I am happy to do that but have no equipment. If you have the capability, please email me.
If one desired to isolate which component(s) of Orabloc may be responsible for seemingly self-assembling or “blowing”, one would inspect each ingredient and see if any lone component does it.
Future studies would then combine added incredients below to see which may re-create the same environment. Here the list of ingredients:
Orabloc™ Ingredients
An EpiPen. This initially seems that it would remove articaine as a causative agent in producing unusual, microscopic objects. However, there could be an interaction between the two.
Normal saline. Virtually every IV bag in the hospital runs on either 0.9% or 0.45% (or “half normal”) saline. You can also obtain a small bottle of normal saline and test that.
Articaine. Pure articaine without epinephrine should be tested.
Hydrochloric acid. You cannot put HCl on a microscope slide, or it would burn a hole. I doubt if any living mechanical thing can live in it, either.
Sodium metabisulfite.
Sodium metabisulfite or sodium pyrosulfite (IUPAC spelling; Br. E. sodium metabisulphite or sodium pyrosulphite) is an inorganic compound of chemical formula Na2S2O5. The substance is sometimes referred to as disodium metabisulfite. It is used as a disinfectant, antioxidant, and preservative agent.[2] When dissolved in water it forms sodium bisulfite.
The above pictogram shows a noted caution that it burns the skin and objects.
Uses
Sodium and potassium metabisulfite have many major and niche uses. It is widely used for preserving food and beverages.
Sodium metabisulfite is added as an excipient to medications which contain adrenaline (epinephrine), in order to prevent the oxidation of adrenaline.[6]For example, it is added to combination drug formulations which contain a local anaesthetic and adrenaline,[6] and to the formulation in epinephrine autoinjectors, such as the EpiPen.[7]This lengthens the shelf life of the formulation,[6] although the sodium metabisulphite reacts with adrenaline, causing it to degrade and form epinephrine sulphonate.[8]
In combination with sodium hydrosulfiteit is used as a rust-stain remover[9]
It is used in photography.[10]
Concentrated sodium metabisulfite can be used to remove tree stumps. Some brands contain 98% sodium metabisulfite, and cause degradation of lignin in the stumps, facilitating removal.[11]
It is also used as an excipient in some tablets, such as paracetamol.
A very important health related aspect of this substance is that it can be added to a blood smear in a test for sickle cell anaemia (and other similar forms of haemoglobin mutation). The substance causes defunct cells to sickle (through a complex polymerisation) hence confirming disease.
It is used as a bleaching agent in the production of coconut cream
It (or liquid SO2) is commonly used as an antimicrobial and antioxidant in winemaking; bottled wine indicates its use with the label "Contains Sulfites" in the US.
It is used as a reducing agent to break sulfide bonds in shrunken items of clothing made of natural fibres, thus allowing the garment to go back to its original shape after washing
It is used as an SO2 source (mixed with air or oxygen) for the destruction of cyanide in commercial gold cyanidationprocesses.
It is used as an SO2 source (mixed with air or oxygen) for the precipitation of elemental gold in chloroauric (aqua regia) solutions.
It is used in the water treatment industry to quench residual chlorine.
It is used in tint etching iron-based metal samples for microstructural analysis.[12][13]
It is used as a fungicide for anti-microbe and mould prevention during shipping of consumer goods such as shoes and clothing. Plastic stickers and packaging (such as Micro-Pak™) containing the anhydrous, sodium metabisulfite solid active ingredient are added prior to shipping. The devices absorb moisture from the atmosphere during shipping and release low levels of sulfur dioxide.[14]
It is used for preserving fruit during shipping.[15]
It is used as a solvent in the extraction of starch from tubers,[16] fruit,[17] and cereal crops.[18][19]
It is used as a pickling agent to treat high pressure reverse osmosis and nanofiltration water desalination membranes for extended storage periods between uses.
Source:
Water. Most probably sterile water, which comes in bottles.
I have a regular microscope, but it is not dark field, nor does it have great resolution. Others who already have the equipment can repeat it; please let us know your interest so that we can follow you, or connect with Will via message on the Substack App.
I would be happy to study it but would need a donation for equipment. If you are interested in helping, please either donate through a one-time coffee, a paid subscription, use my ministry donation link, or email me directly. There are popular microscopes with classes available from $5,000-$10,000. Thank you.
Thank you for reading my writings!






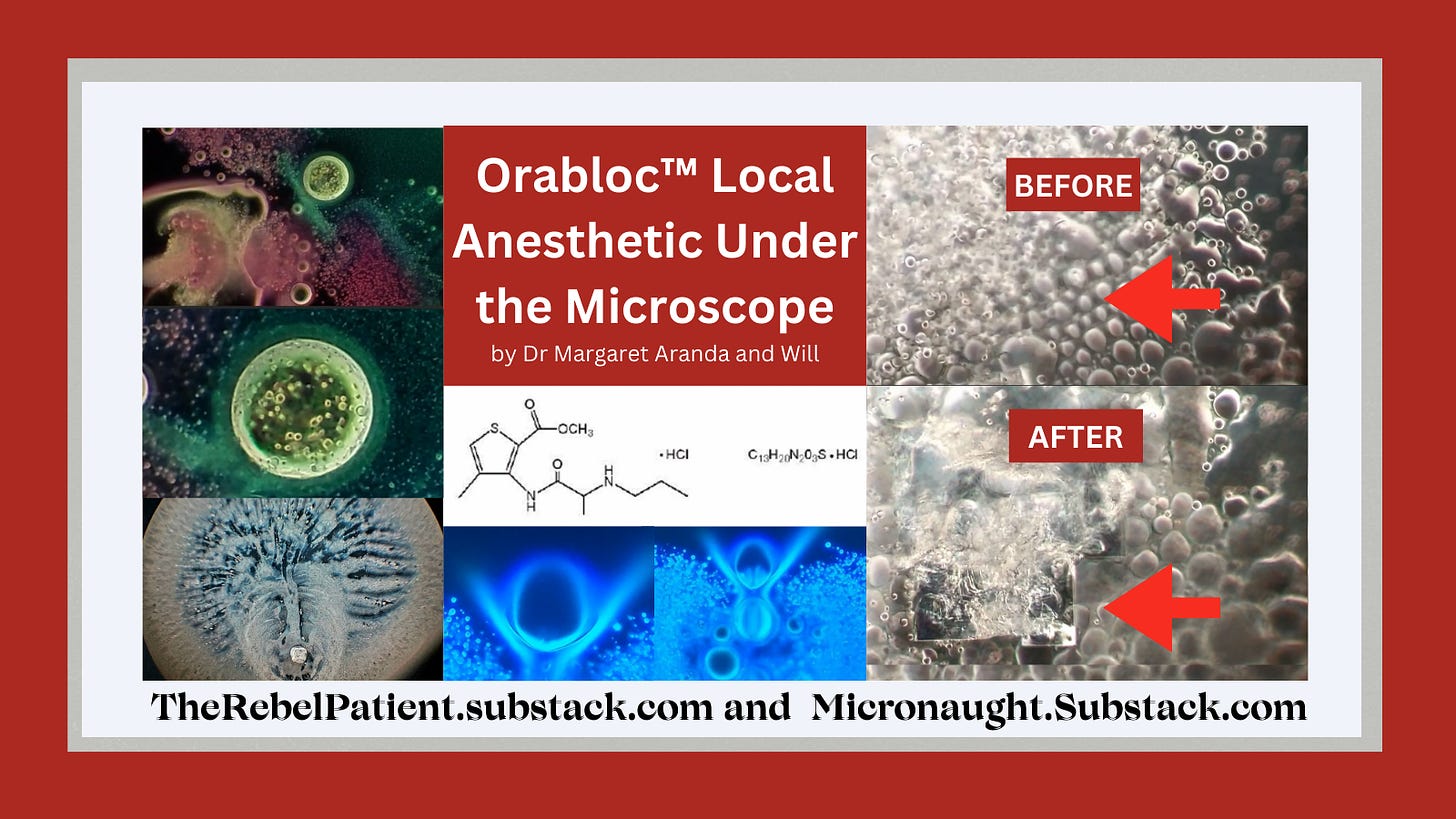


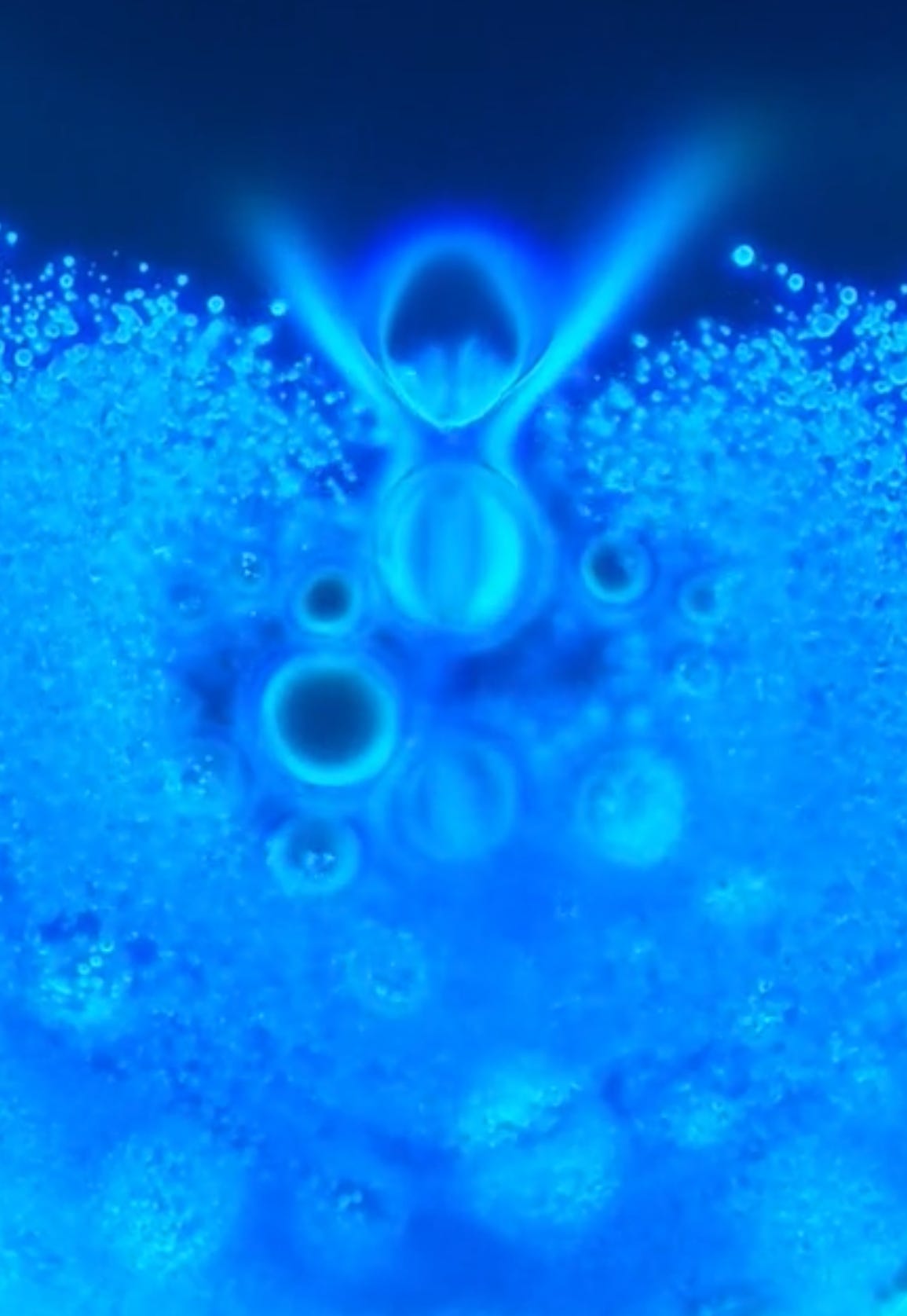
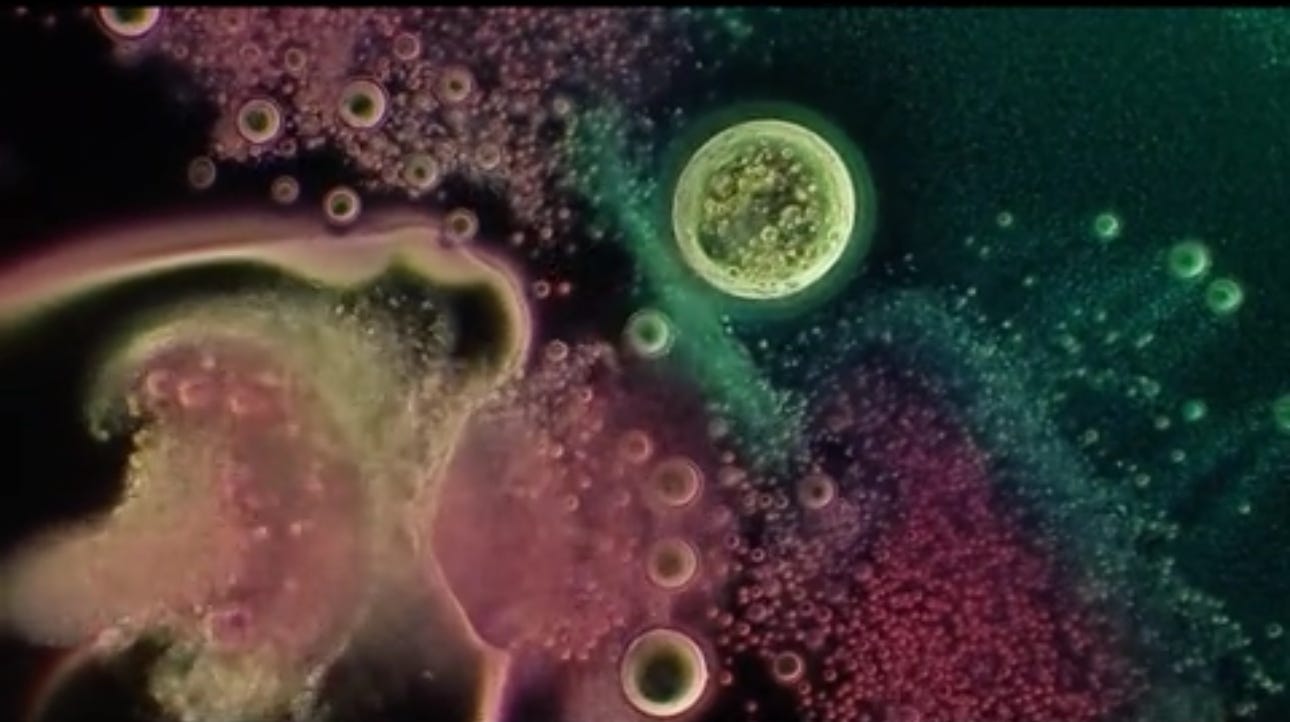
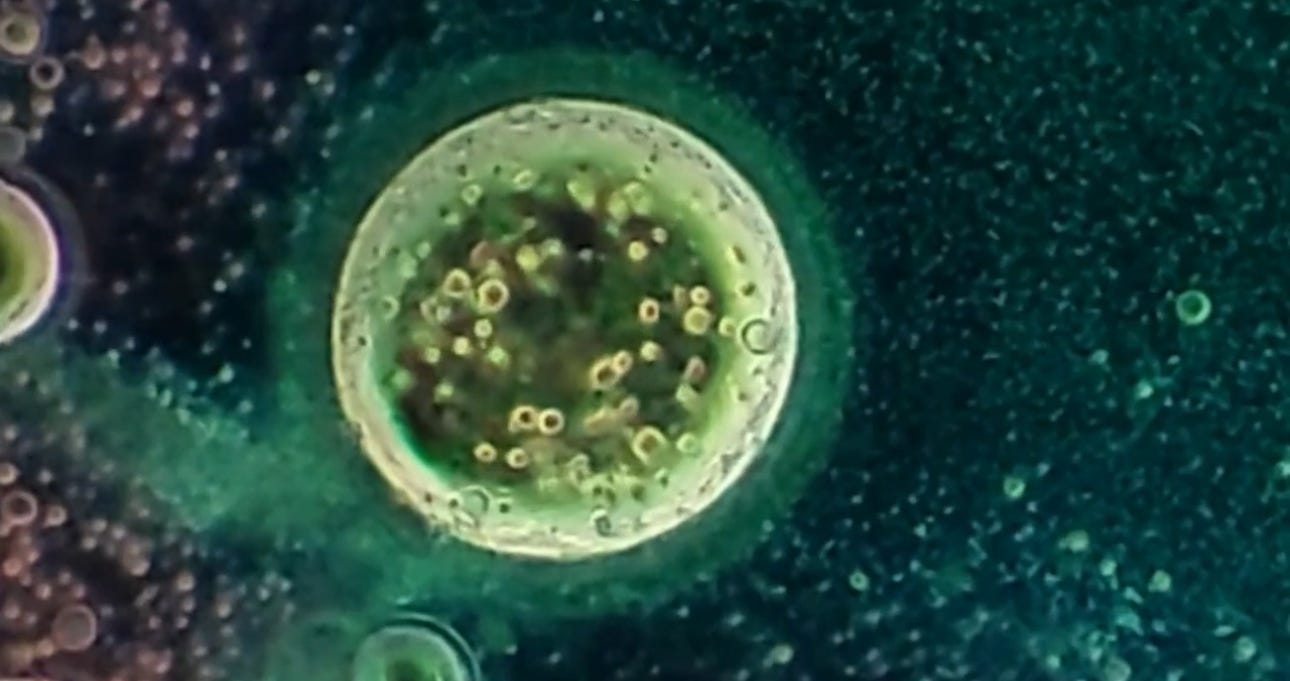
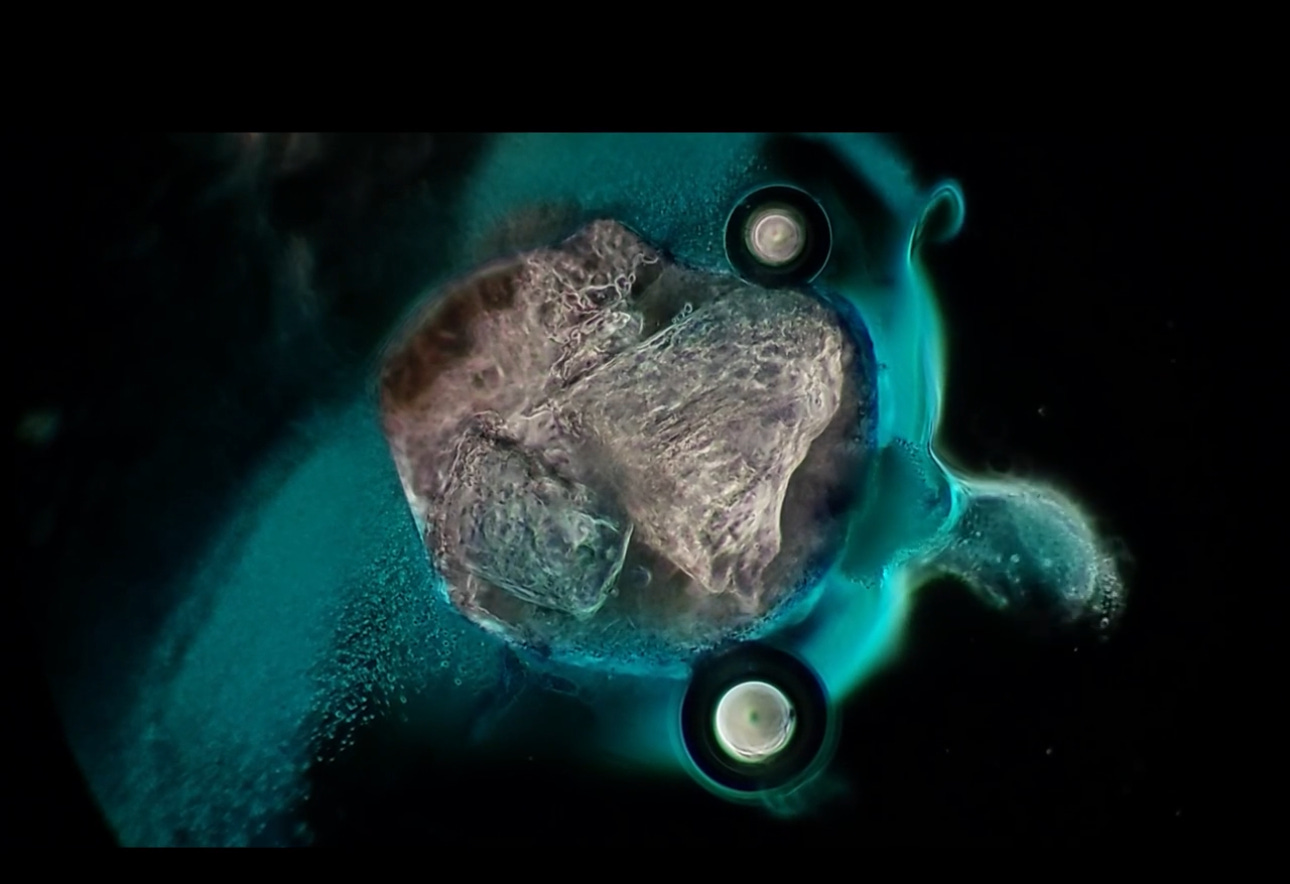
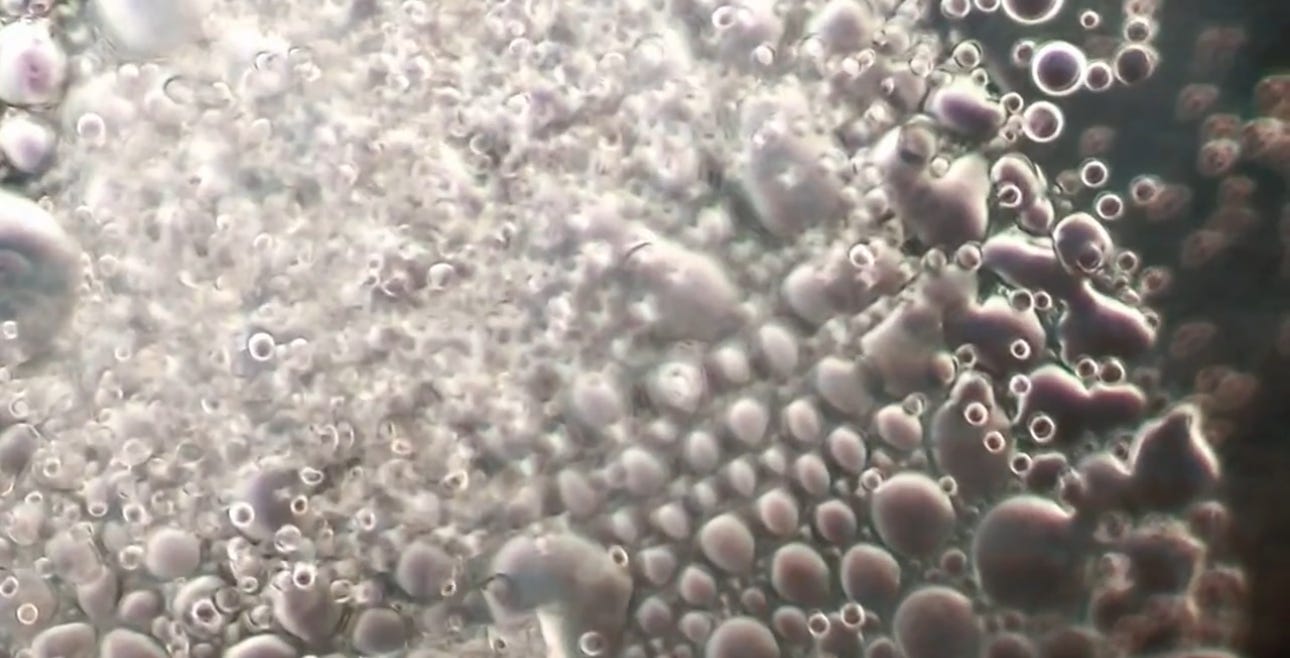
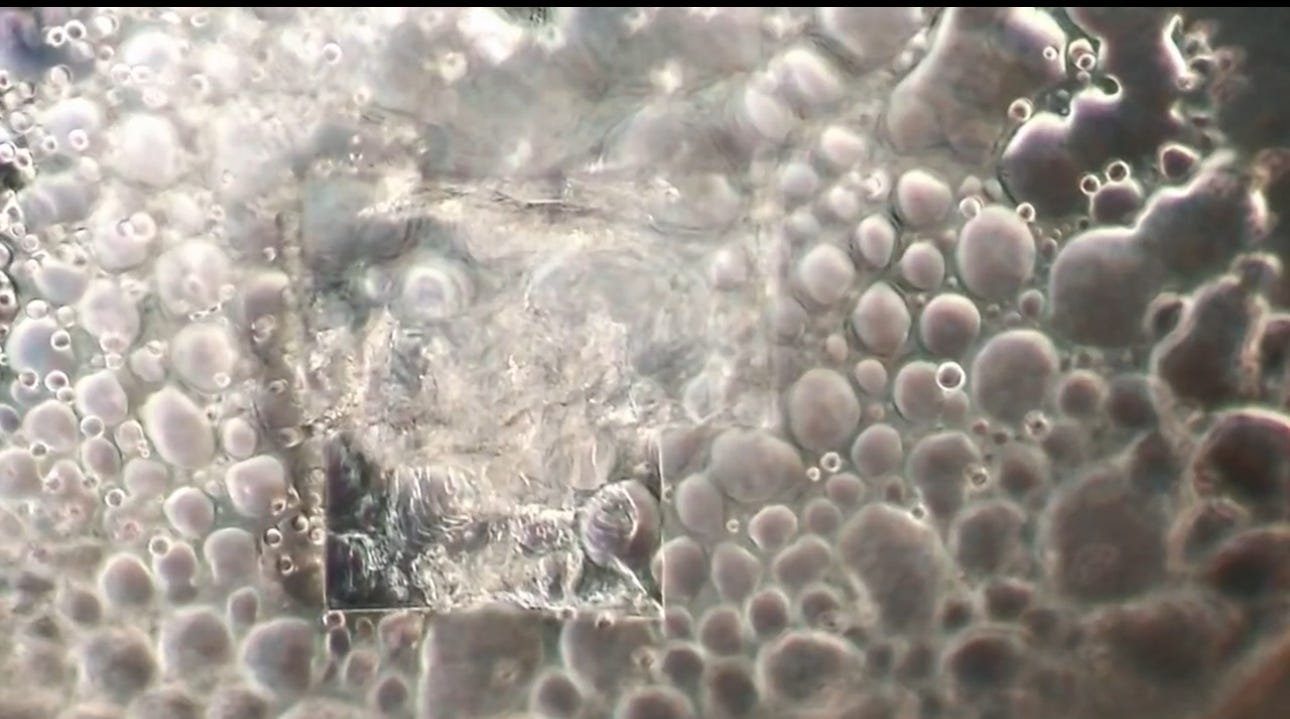



There is observable reiterative symmetry and movement in the architecture and its artifacts akin to electromagnetic conductivity. Not saying it is.
You know this needs to be addressed. I had a tooth extraction a couple months ago and I debated about talking to the surgeon about it. I ended up not saying anything because if he knows he is using tainted anesthetic then is he really going to admit it? Do dentists even know how to check this stuff under a microscope? I doubt it. But somebody that does needs to do a study of it. Nobody likes going to the dentist but if you have an emergency with a painful tooth, you will do about anything to get it to go away!!