Zinc Research in 2010 Shows Lab Suppression of Coronavirus by Zinc Ionophore Plus Zinc, per Zelenko Protocol, Yet Silence by Baric, Authors, Fauci, CDC, NIH, FDA
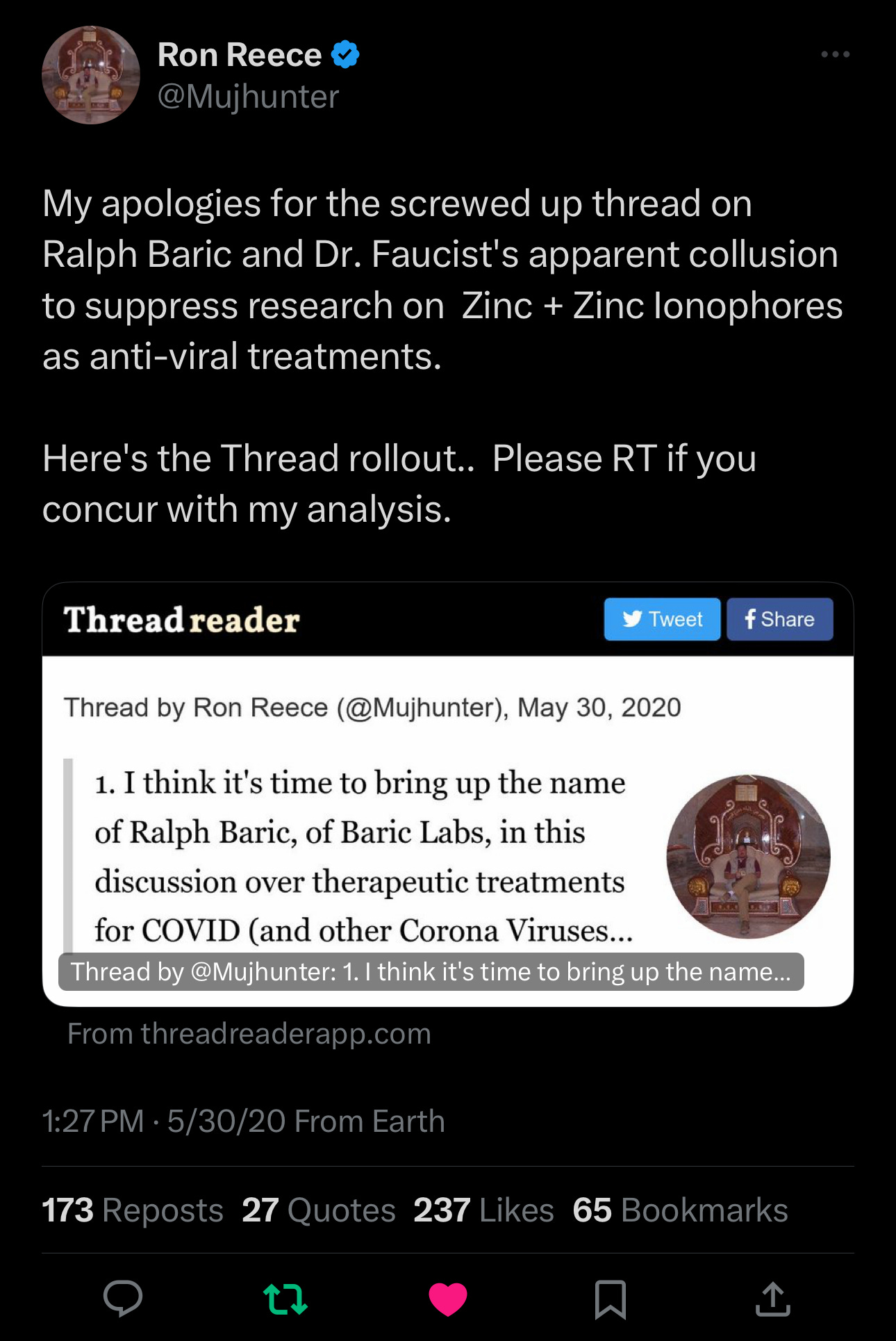
These Research Findings Were Declared by Some Like Ron Reese @Mujhunter on Twitter/X on May 30, 2020 - He Pleaded to A Group of Medical Freedom Doctors Yesterday and This is My Reply
We Should Have Known
Now we need to look up previous research and check it for previous knowledge and in today's world, also check it for evil intentions and suppression of healing compounds in favor of Big Pharma.
Source: https://x.com/mujhunter/status/1266798397605871616?s=46
Levels of Suppression and Control
Ron Reese ties all these together:
Baric’s Research Shows Zinc + Drugs like Hydroxychloroquine (HCQ) Work Against Coronavirus - in 2010.
The Zelenko Protocol Issues Rx for HCQ, EGCG, Zinc, Vitamin C.
Baric and Fauci Collaborate on Wuhan Research.
Baric Works on Remdesivir.
Remdesivir: The only FDA-Approved Drug for Covid.
All done to suppress a known treatment and substitute it for a money-making scheme.
The Tweet That Started It
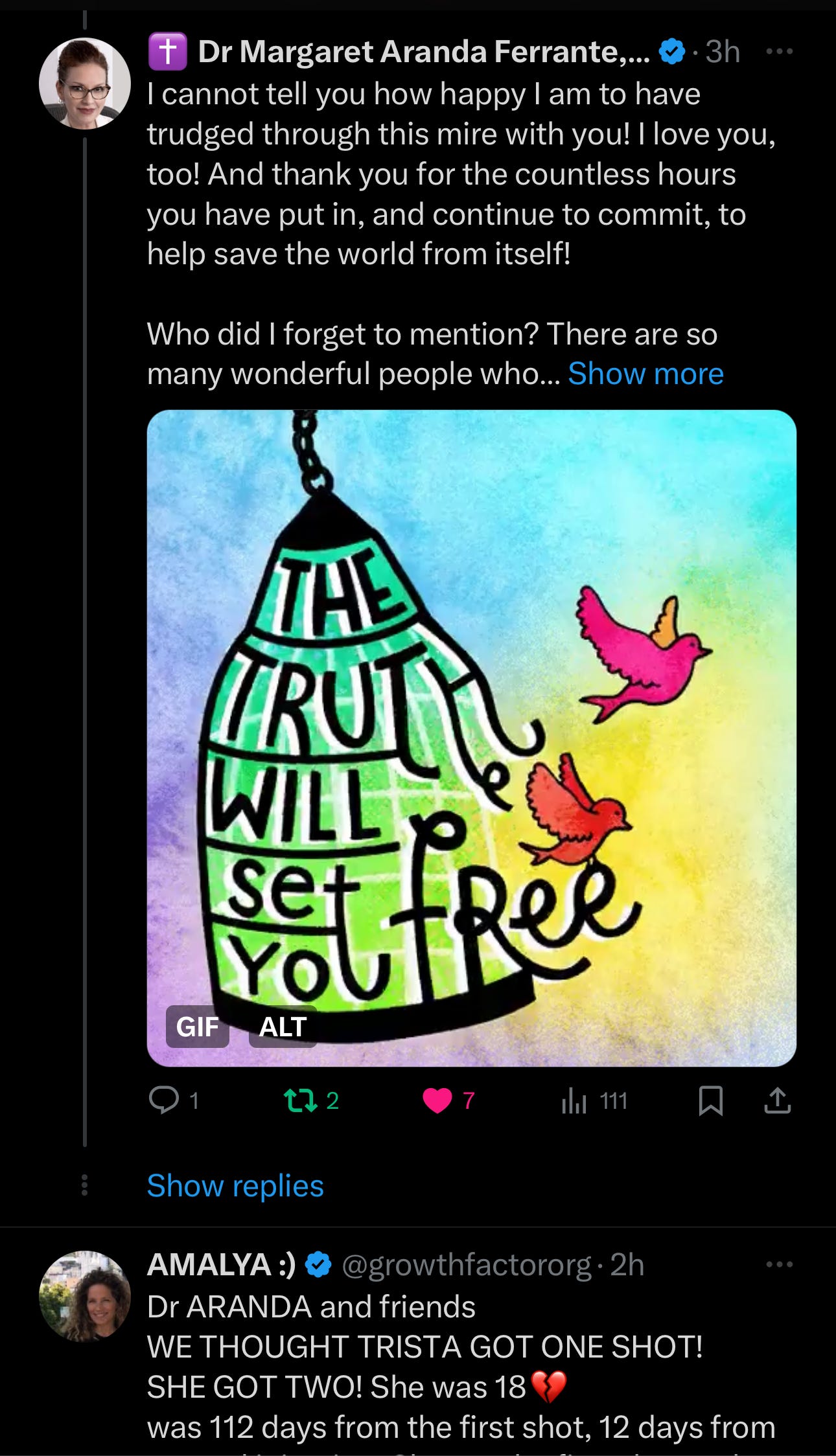
This article began innocently enough last night at 6:30 pm, with a tweet by Dr. Ben Marble listing some medical freedom fighters. I replied with thanks and then listed many more people who fought with us.
Dr. Marble’s tweet: https://x.com/benmarble_md/status/1751764443283832881?s=46
I was happy the conversations were so active, especially as it was getting late. I still tried to think of all the people that have helped… the list grew. And I didn't want to leave anyone out.
I've made many friends over the years and we all stick together, having Zoomed with both Amalya and Thomas for my Rumble. Amayla also interviewed me on my Near-Death Experience. For those who don't know, I had a traumatic brain injury and was bedridden for twelve years before being healed by God's miracle.
(I paused here to respond to Thomas, who is in the UK.)
I missed it at first but saw a different plea from someone unfamiliar to me. I responded, then wrote this article.
I immediately saw that too much time had gone by for this to have remained untouched:
I clicked his link, which led to this tweet:
And I promised to investigate.
The Above Link Goes To A Paper I Never Saw
First, understand that a “zinc ionophore” opens the door to a virus outer wall. Then the zinc goes in and stops the virus from multiplying.
This is how hydroxychloroquine, quercetin, green tea, EGCG, and doxycycline all work: they are zinc ionophores.
The Original Paper Showing Zinc + “PT”, a Test-tube Zinc Ionophore, Stopped Coronavirus from Multiplying:
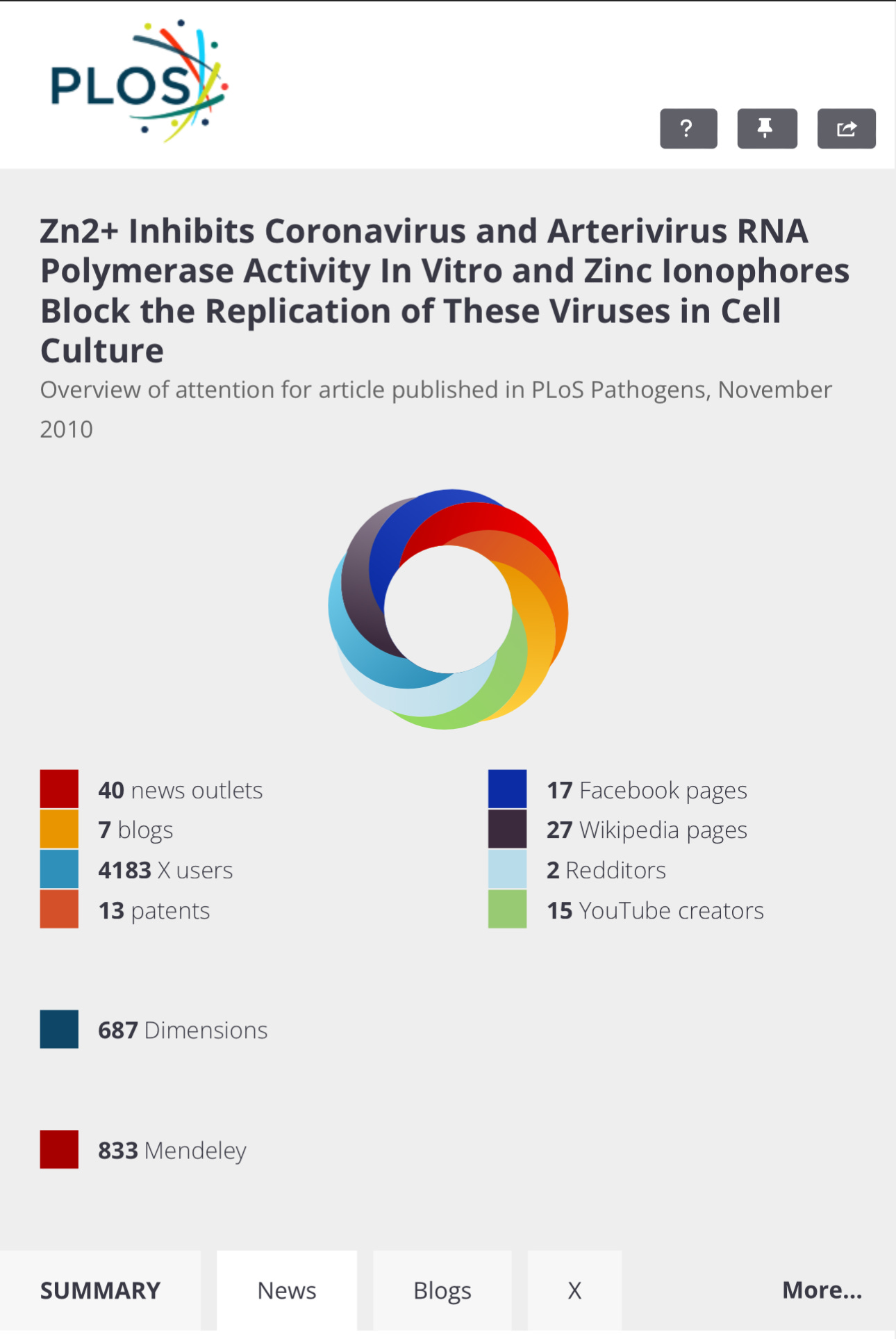
Source: https://journals.plos.org/plospathogens/article?id=10.1371/journal.ppat.1001176
The connection between a hydroxychloroquine + zinc and the inhibition of virus replication was made by Baric in 2010.
There were other authors who knew this as well. Dr. Zelenko figured it out but it seems that none of us knew, or we would have shouted it from the rooftops.
Ron Reese was right. Had this information been known, millions would have been helped from virtually any virus, including Coronavirus, Poliovirus, influenza virus. Baric et al even cite previous research showing zinc ionophores plus zinc can inhibit virus replication for “rhinoviruses, foot-and-mouth disease virus, coxsackievirus, and mengovirus”.
Pause: Polio can be helped with zinc + hydroxychloroquine, too!
Paper Received: May 17, 2010; Accepted: October 1, 2010; Published: November 4, 2010.
Summary: In a lab, zinc plus HCQ stops the Coronavirus (SARS-CoV) from Multiplying
With full permission to copy the article and credit the authors, here is the full text.
Abstract
Increasing the intracellular Zn2+ concentration with zinc-ionophores like pyrithione (PT) can efficiently impair the replication of a variety of RNA viruses, including poliovirus and influenza virus. For some viruses this effect has been attributed to interference with viral polyprotein processing. In this study we demonstrate that the combination of Zn2+ and PT at low concentrations (2 µM Zn2+ and 2 µM PT) inhibits the replication of SARS-coronavirus (SARS-CoV) and equine arteritis virus (EAV) in cell culture. The RNA synthesis of these two distantly related nidoviruses is catalyzed by an RNA-dependent RNA polymerase (RdRp), which is the core enzyme of their multiprotein replication and transcription complex (RTC). Using an activity assay for RTCs isolated from cells infected with SARS-CoV or EAV—thus eliminating the need for PT to transport Zn2+ across the plasma membrane—we show that Zn2+ efficiently inhibits the RNA-synthesizing activity of the RTCs of both viruses. Enzymatic studies using recombinant RdRps (SARS-CoV nsp12 and EAV nsp9) purified from E. coli subsequently revealed that Zn2+ directly inhibited the in vitro activity of both nidovirus polymerases. More specifically, Zn2+ was found to block the initiation step of EAV RNA synthesis, whereas in the case of the SARS-CoV RdRp elongation was inhibited and template binding reduced. By chelating Zn2+ with MgEDTA, the inhibitory effect of the divalent cation could be reversed, which provides a novel experimental tool for in vitro studies of the molecular details of nidovirus replication and transcription.
Author Summary
Positive-stranded RNA (+RNA) viruses include many important pathogens. They have evolved a variety of replication strategies, but are unified in the fact that an RNA-dependent RNA polymerase (RdRp) functions as the core enzyme of their RNA-synthesizing machinery. The RdRp is commonly embedded in a membrane-associated replication complex that is assembled from viral RNA, and viral and host proteins. Given their crucial function in the viral replicative cycle, RdRps are key targets for antiviral research. Increased intracellular Zn2+ concentrations are known to efficiently impair replication of a number of RNA viruses, e.g. by interfering with correct proteolytic processing of viral polyproteins. Here, we not only show that corona- and arterivirus replication can be inhibited by increased Zn2+ levels, but also use both isolated replication complexes and purified recombinant RdRps to demonstrate that this effect may be based on direct inhibition of nidovirus RdRps. The combination of protocols described here will be valuable for future studies into the function of nidoviral enzyme complexes.
Citation: te Velthuis AJW, van den Worm SHE, Sims AC, Baric RS, Snijder EJ, van Hemert MJ (2010) Zn2+Inhibits Coronavirus and Arterivirus RNA Polymerase Activity In Vitro and Zinc Ionophores Block the Replication of These Viruses in Cell Culture. PLoS Pathog 6(11): e1001176. doi:10.1371/journal.ppat.1001176
Editor: Raul Andino, University of California San Francisco, United States of America
Received: May 17, 2010; Accepted: October 1, 2010; Published: November 4, 2010
Copyright: © 2010 te Velthuis et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Funding: This work was supported by the Netherlands Organization for Scientific Research (NWO) with grants from the Council for Chemical Sciences (NWO-CW grant 700.55.002 and 700.57.301) and an NWO Toptalent grant (021.001.037). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Competing interests: The authors have declared that no competing interests exist.
Introduction
Zinc ions are involved in many different cellular processes and have proven crucial for the proper folding and activity of various cellular enzymes and transcription factors. Zn2+ is probably an important cofactor for numerous viral proteins as well. Nevertheless, the intracellular concentration of free Zn2+ is maintained at a relatively low level by metallothioneins, likely due to the fact that Zn2+ can serve as intracellular second messenger and may trigger apoptosis or a decrease in protein synthesis at elevated concentrations [1], [2], [3]. Interestingly, in cell culture studies, high Zn2+ concentrations and the addition of compounds that stimulate cellular import of Zn2+, such as hinokitol (HK), pyrrolidine dithiocarbamate (PDTC) and pyrithione (PT), were found to inhibit the replication of various RNA viruses, including influenza virus [4], respiratory syncytial virus [5] and several picornaviruses [6], [7], [8], [9], [10], [11]. Although these previous studies provided limited mechanistic information, this suggests that intracellular Zn2+ levels affect a common step in the replicative cycle of these viruses.
In cell culture, PT stimulates Zn2+ uptake within minutes and inhibits RNA virus replication through a mechanism that has only been studied in reasonable detail for picornaviruses [11], [12]. In vitro studies with purified rhinovirus and poliovirus 3C proteases revealed that protease activity was inhibited by Zn2+ [13], [14], which is in line with the inhibition of polyprotein processing by zinc ions that was observed in cells infected with human rhinovirus and coxsackievirus B3 [11]. The replication of segmented negative-strand RNA viruses such as influenza virus, however, does not depend on polyprotein processing and the effect of PDTC-mediated Zn2+ import was therefore hypothesized to result from inhibition of the viral RNA-dependent RNA polymerase (RdRp) and cellular cofactors [4]. Moreover, an inhibitory effect of Zn2+ on the activity of purified RdRps from rhinoviruses and hepatitis C virus was noted, but not investigated in any detail [15], [16].
Details on the effect of zinc ions are currently largely unknown for nidoviruses. This large group of positive-strand RNA (+RNA) viruses includes major pathogens of humans and livestock, such as severe acute respiratory syndrome coronavirus (SARS-CoV), other human coronaviruses, the arteriviruses equine arteritis virus (EAV), and porcine reproductive and respiratory syndrome virus (PRRSV) [17], [18]. The common ancestry of nidoviruses is reflected in their similar genome organization and expression strategy, and in the conservation of a number of key enzymatic functions in their large replicase polyproteins [19]. A hallmark of the corona- and arterivirus replicative cycle is the transcription of a 5′- and 3′-coterminal nested set of subgenomic (sg) mRNAs from which the viral structural and accessory protein genes are expressed [20], [21].
Analogous to picornaviruses [13], [22], zinc ions were demonstrated to inhibit certain proteolytic cleavages in the processing of the coronavirus replicase polyproteins in infected cells and cell-free systems [23], [24]. In this study we report that the zinc-ionophore pyrithione (PT) in combination with Zn2+ is a potent inhibitor of the replication of SARS-coronavirus (SARS-CoV) and equine arteritis virus (EAV) in cell culture. To assess whether - besides a possible effect on proteolytic processing - nidovirus RTC subunits and RNA synthesis are directly affected by Zn2+, we employed in vitro systems for SARS-CoV and EAV RNA synthesis that are based on membrane-associated RTCs isolated from infected cells (from here on referred to as RTC assays) [25], [26]. In addition, we used in vitrorecombinant RdRp assays to directly study the effect of zinc ions on the RdRps of SARS-CoV and EAV [27], [28].
Using these independent in vitro approaches, we were able to demonstrate that Zn2+ directly impairs nidovirus RNA synthesis, since it had a strong inhibitory effect in both RTC and RdRp assays. Interestingly, the Zn2+-mediated inhibition could be reversed through the addition of a Zn2+ chelator (MgEDTA). We therefore applied this compound to stop and restart the in vitroRNA-synthesizing activity at will. This convenient tool allowed us to study various mechanistic aspects of arteri- and coronavirus RNA synthesis in more detail. Additionally, the zinc-mediated inhibition of nidovirus RNA synthesis described here may provide an interesting basis to further explore the use of zinc-ionophores in antiviral therapy.
Results
Zinc and pyrithione inhibit nidovirus replication in vivo
Zinc ions are involved in many different cellular processes, but the concentration of free Zn2+ is maintained at a relatively low level by metallothioneins [1]. Zn2+ and compounds that stimulate import of Zn2+into cells, such as PT, were previously found to inhibit replication of several picornaviruses, including rhinoviruses, foot-and-mouth disease virus, coxsackievirus, and mengovirus in cell culture [6], [7], [8], [9], [10], [11]. To determine whether Zn2+ has a similar effect on nidoviruses, we investigated the effect of PT and Zn2+ on the replication of EAV and SARS-CoV in Vero-E6 cells, using reporter viruses that express green fluorescent proteins (GFP), i.e., EAV-GFP [29] and SARS-CoV-GFP [30]. EAV-GFP encodes an N-terminal fusion of GFP to the viral nonstructural protein 2 (nsp2), one of the cleavage products of the replicase polyproteins, and thus provides a direct readout for translation of the replicase gene. In SARS-CoV-GFP, reporter expression occurs from sg mRNA 7, following the replacement of two accessory protein-coding genes (ORFs 7a and 7b) that are dispensable for replication in cell culture.
We first assessed the cytotoxicity of a range of PT concentrations (0–32 µM) in the presence of 0 to 8 µM ZnOAc2. Treatment with PT of concentrations up to 32 µM in combination with <4 µM ZnOAc2 did not reduce the viability of mock-infected cells after 18 h (Fig. 1A), as measured by the colorimetric MTS (3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium) viability assay. As elevated Zn2+ concentrations are known to inhibit cellular translation, we also used metabolic labeling with 35S-methionine to assess the effect of PT and Zn2+ on cellular protein synthesis. Incubation of Vero-E6 cells for 18 h with the combinations of PT and Zn2+mentioned above, followed by a 2-h metabolic labeling, revealed no change in overall cellular protein synthesis when the concentration of ZnOAc2 was <4 µM (data not shown).
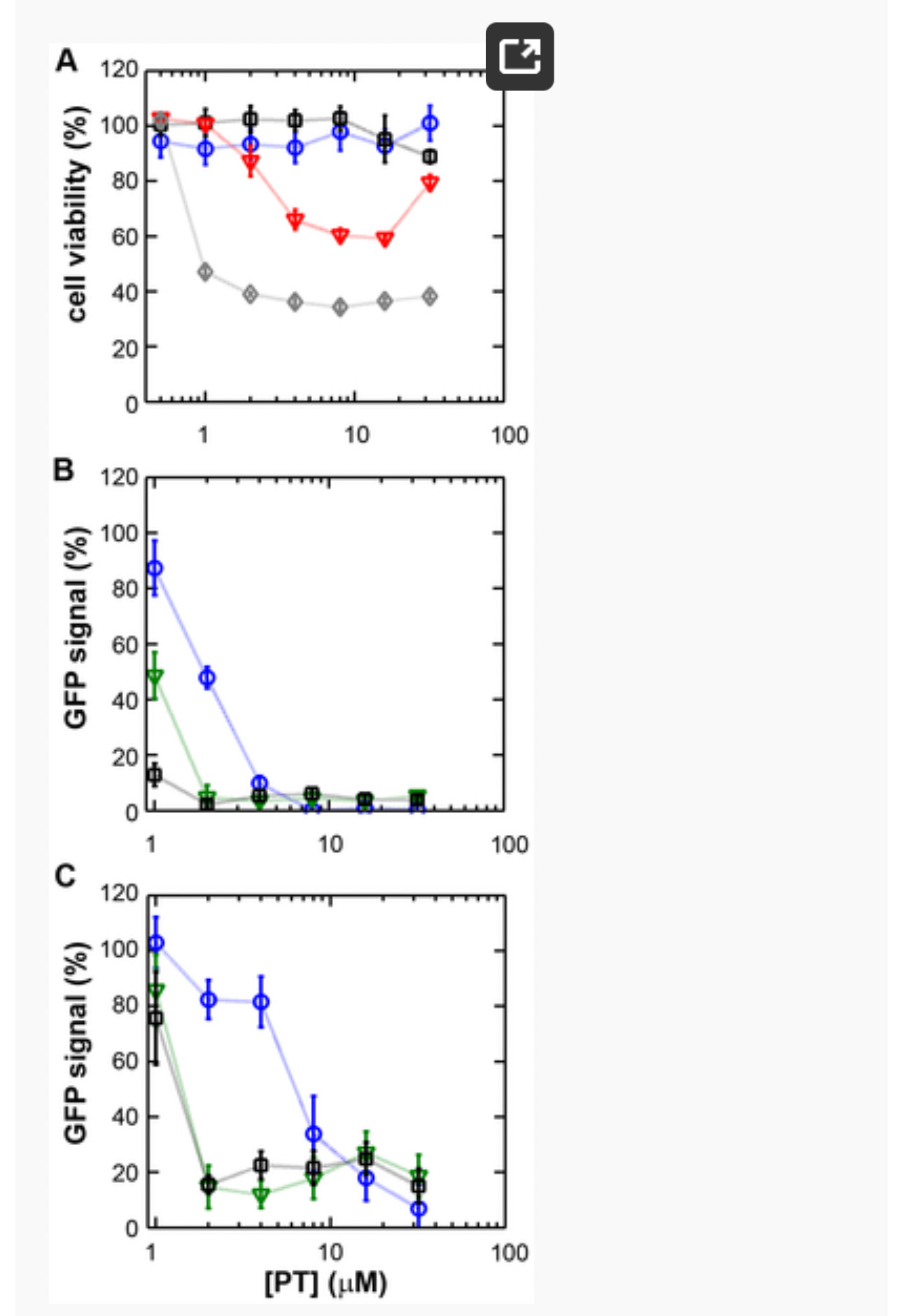
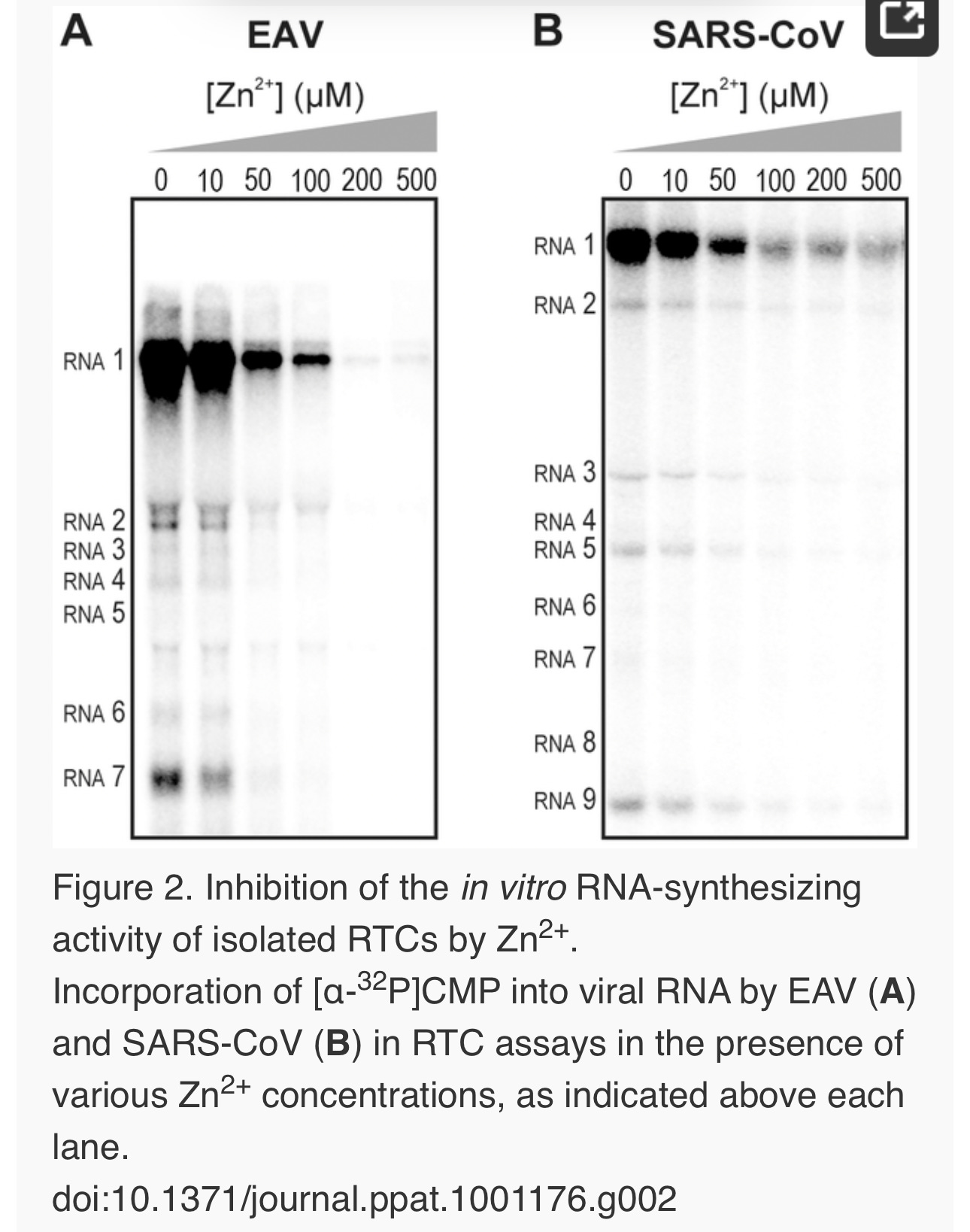
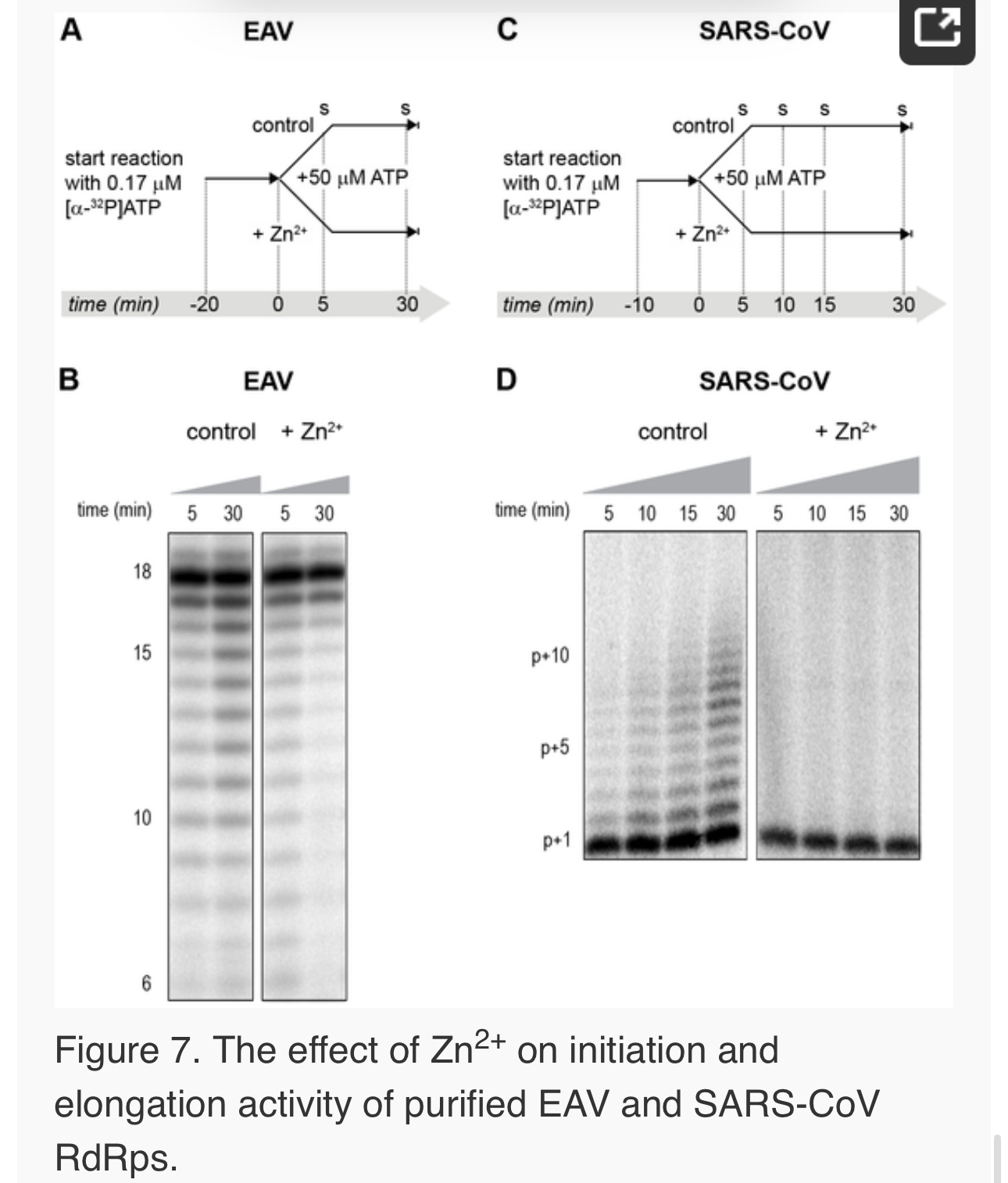
Figure 1. The zinc ionophore pyrithione inhibits nidovirus replication in cell culture.
(A) Cytotoxicity of PT in Vero-E6 cells in the absence (blue circles) or presence of 2 (black squares), 4 (red triangles), or 8 µM (gray diamonds) ZnOAc2 as determined by the MTS assay after 18 hours of exposure. (B) Dose-response curves showing the effect of PT and Zn2+ on the GFP fluorescence in Vero-E6 cells infected with a GFP-expressing EAV reporter strain at 17 h p.i. Data were normalized to GFP expression in infected, untreated control cultures (100%). The different Zn2+ concentrations added to the medium were 0 (blue circles), 1 (green triangles), or 2 µM ZnOAc2 (black squares). (C) Effect of PT and Zn2+ on the GFP fluorescence in Vero-E6 cells infected with a GFP-expressing SARS-CoV reporter strain at 17 h p.i. Data were normalized to GFP expression in infected untreated control cells (100%). Colors for different Zn2+ concentrations as in Fig. 1B. Error bars indicate the standard deviation (n = 4). doi:10.1371/journal.ppat.1001176.g001
Using these non-cytotoxic conditions we subsequently tested the effect of PT and ZnOAc2 on EAV-GFP and SARS-CoV-GFP replication. To this end, Vero-E6 cells in 96-well plates were infected with a multiplicity of infection (m.o.i.) of 4. One hour post infection (h p.i.), between 0 and 32 µM of PT and 0, 1, or 2 µM ZnOAc2were added to the culture medium. At 17 h p.i., a time point at which GFP expression in untreated infected cells reaches its maximum for both viruses, cells were fixed, and GFP fluorescence was quantified.
The reporter gene expression of both SARS-CoV-GFP and EAV-GFP was already significantly inhibited in a dose-dependent manner by the addition of PT alone (Fig. 1B and C). This effect was significantly enhanced when 2 µM of Zn2+ was added to the medium. We found that addition of ZnOAc2 alone also reduced virus replication, but only at levels that were close to the 50% cytotoxicity concentration (CC50) of ZnOAc2 in Vero-E6 cells (∼70 µM, data not shown). This is likely due to the poor solubility of Zn2+ in phosphate-containing medium and the inefficient uptake of Zn2+ by cells in the absence of zinc-ionophores. The combination of 2 µM PT and 2 µM ZnOAc2 resulted in a 98±1% and 85±3% reduction of the GFP signal for EAV-GFP and SARS-CoV-GFP, respectively. No cytotoxicity was observed for this combination of PT and ZnOAc2 concentrations. From the dose-response curves in Fig. 1, a CC50 value of 82 µM was calculated for PT in the presence of 2 µM zinc. Half maximal inhibitory concentrations (IC50) of 1.4 µM and 0.5 µM and selectivity indices of 59 and 164 were calculated for SARS-CoV and EAV, respectively.
Zn2+ reversibly inhibits the RNA-synthesizing activity of isolated nidovirus RTCs
We previously developed assays to study the in vitroRNA-synthesizing activity of RTCs isolated from cells infected with SARS-CoV or EAV [25], [26]. In these RTC assays [α-32P]CMP is incorporated into both genomic (replication) and sg mRNA (transcription) (Fig. 2). This allowed us to monitor the synthesis of the same viral RNA molecules that can be detected by hybridization of RNA from nidovirus-infected cells. A benefit of these assays is that the activity does not depend on continued protein synthesis and that it allows us to study viral RNA synthesis independent of other aspects of the viral replicative cycle [26]. To investigate whether the inhibitory effect of PT and zinc ions on nidovirus replication in cell culture is reflected in a direct effect of Zn2+ on viral RNA synthesis, we tested the effect of Zn2+ addition on RTC activity. For both EAV (Fig. 2A) and SARS-CoV (Fig. 2B), a dose-dependent decrease in the amount of RNA synthesized was observed when ZnOAc2 was present. For both viruses, a more than 50% reduction of overall RNA-synthesis was observed at a Zn2+ concentration of 50 µM, while less than 5% activity remained at a Zn2+concentration of 500 µM. Both genome synthesis and sg mRNA production were equally affected.
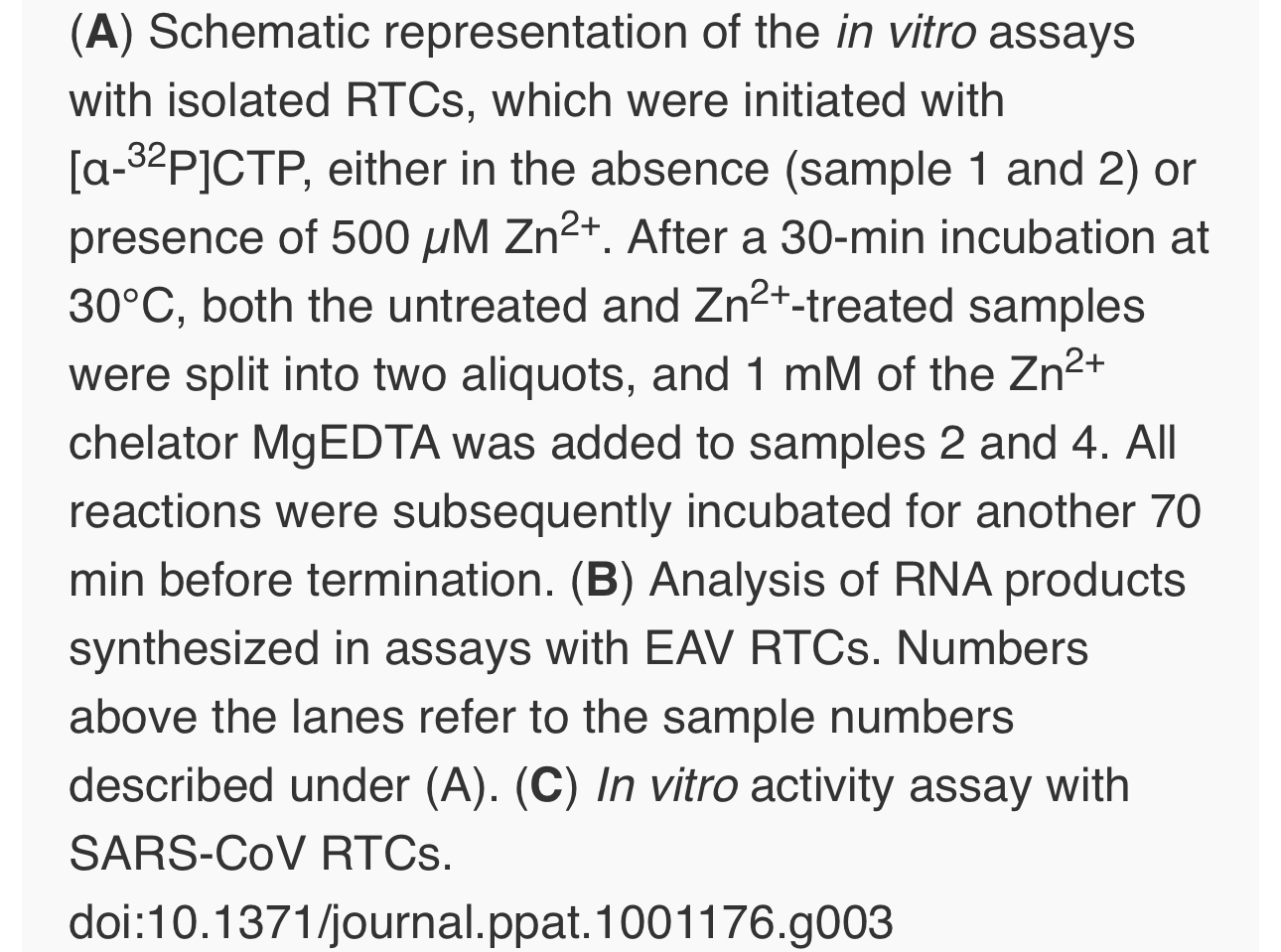
To test whether the inhibition of RTC activity by Zn2+was reversible, RTC reactions were started in the presence or absence of 500 µM Zn2+. After 30 min, these reactions were split into two aliquots and magnesium-saturated EDTA (MgEDTA) was added to one of the tubes to a final concentration of 1 mM (Fig. 3A). We used MgEDTA as Zn2+ chelator in these in vitroassays, because it specifically chelates Zn2+ while releasing Mg2+, due to the higher stability constant of the ZnEDTA complex. Uncomplexed EDTA inhibited RTC activity in all reactions (data not shown), most likely by chelating the Mg2+ that is crucial for RdRp activity [27], [28], whereas MgEDTA had no effects on control reactions without Zn2+ (Fig. 3B, compare lane 1 and 2). As shown in Fig. 2, the EAV RTC activity that was inhibited by Zn2+ (Fig. 3B&C, lane 3) could be restored by the addition of MgEDTA (Fig. 3B, lane 4) to a level observed for control reactions without Zn2+ (Fig. 3B, lane 1). Compared to the untreated control, the EAV RTC assay produced approximately 30% less RNA, which was consistent with the 30% shorter reaction time after the addition of the MgEDTA (100 versus 70 min for lanes 1 and 4, respectively). Surprisingly, SARS-CoV RTC assays that were consecutively supplemented with Zn2+ and MgEDTA incorporated slightly more [α-32P]CMP compared to untreated control reactions (Fig. 3C; compare lane 1 and 4). This effect was not due to chelation of the Zn2+already present in the post-nuclear supernatant (PNS) of SARS-CoV-infected cells, as this increase was not observed when MgEDTA was added to a control reaction without additional Zn2+ (Fig. 3C, lane 2).
Zinc ions affect the in vitro activity of recombinant nidovirus RdRps
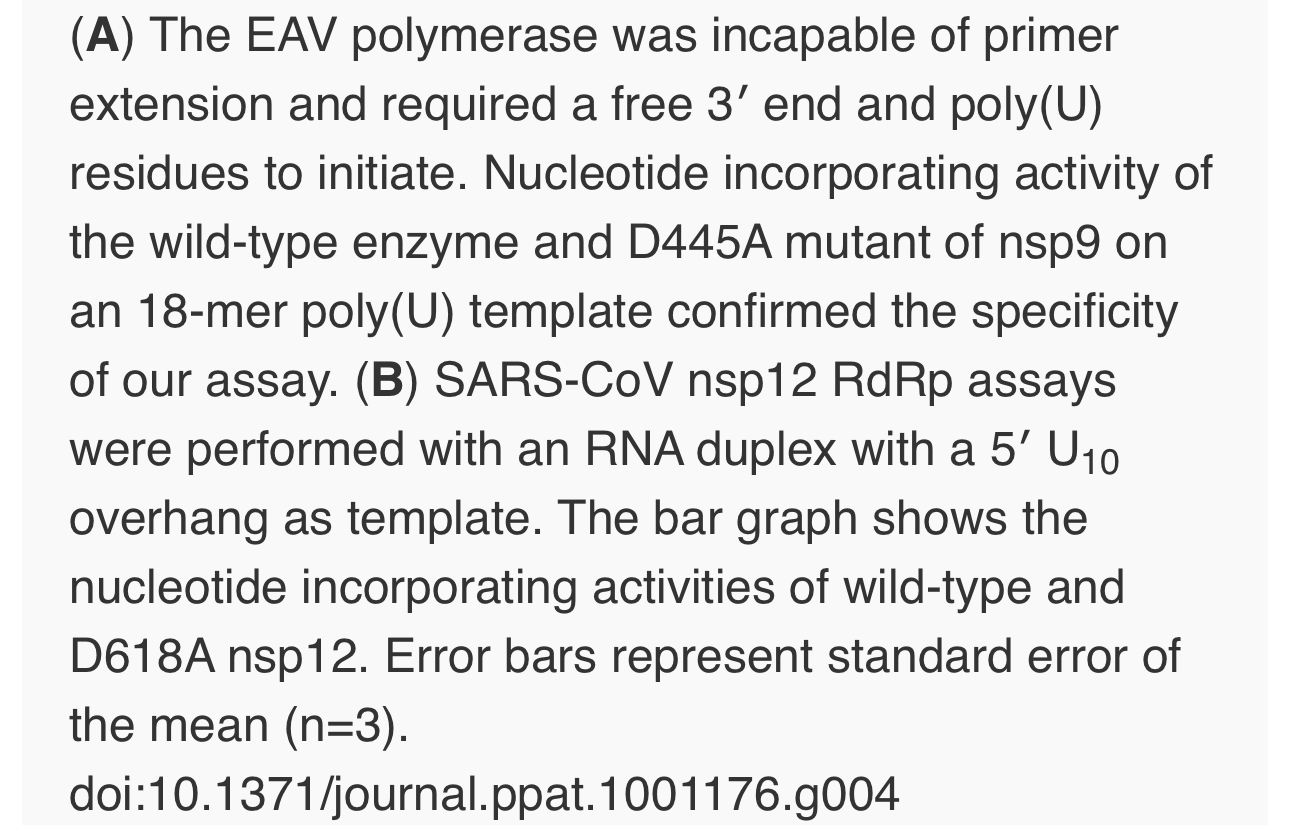
To establish whether inhibition of RTC activity might be due to a direct effect of Zn2+ on nidovirus RdRp activity, we tested the effect of Zn2+ on the activity of the purified recombinant RdRps of SARS-CoV (nsp12) and EAV (nsp9) using previously developed RdRp assays [27], [28]. Using an 18-mer polyU template, the EAV RdRp incorporated [α-32P]AMP into RNA products of up to 18 nt in length (Fig. 4A). Initiation was de novo, which is in line with previous observations and the presence of a conserved priming loop in the nsp9 sequence [28]. Unlike the EAV RdRp nsp9, the in vitroactivity of the SARS-CoV RdRp nsp12 - which lacks a priming loop - was shown to be strictly primer-dependent [27]. Thus, to study the RdRp activity of SARS-CoV nsp12, a primed polyU template was used (Fig. 4B), thereby allowing us to sample [α-32P]AMP incorporation as described previously [27]. As specificity controls, we used the previously described SARS-CoV nsp12 mutant D618A [27], which contains an aspartate to alanine substitution in motif A of the RdRp active site, and EAV nsp9-D445A, in which we engineered an aspartate to alanine substitution at the corresponding site of EAV nsp9 [28], [31]. Both mutant RdRps showed greatly reduced [α-32P]AMP incorporation in our assays (Fig. 4), confirming once again that the radiolabeled RNA products derived from nidovirus RdRp activity.
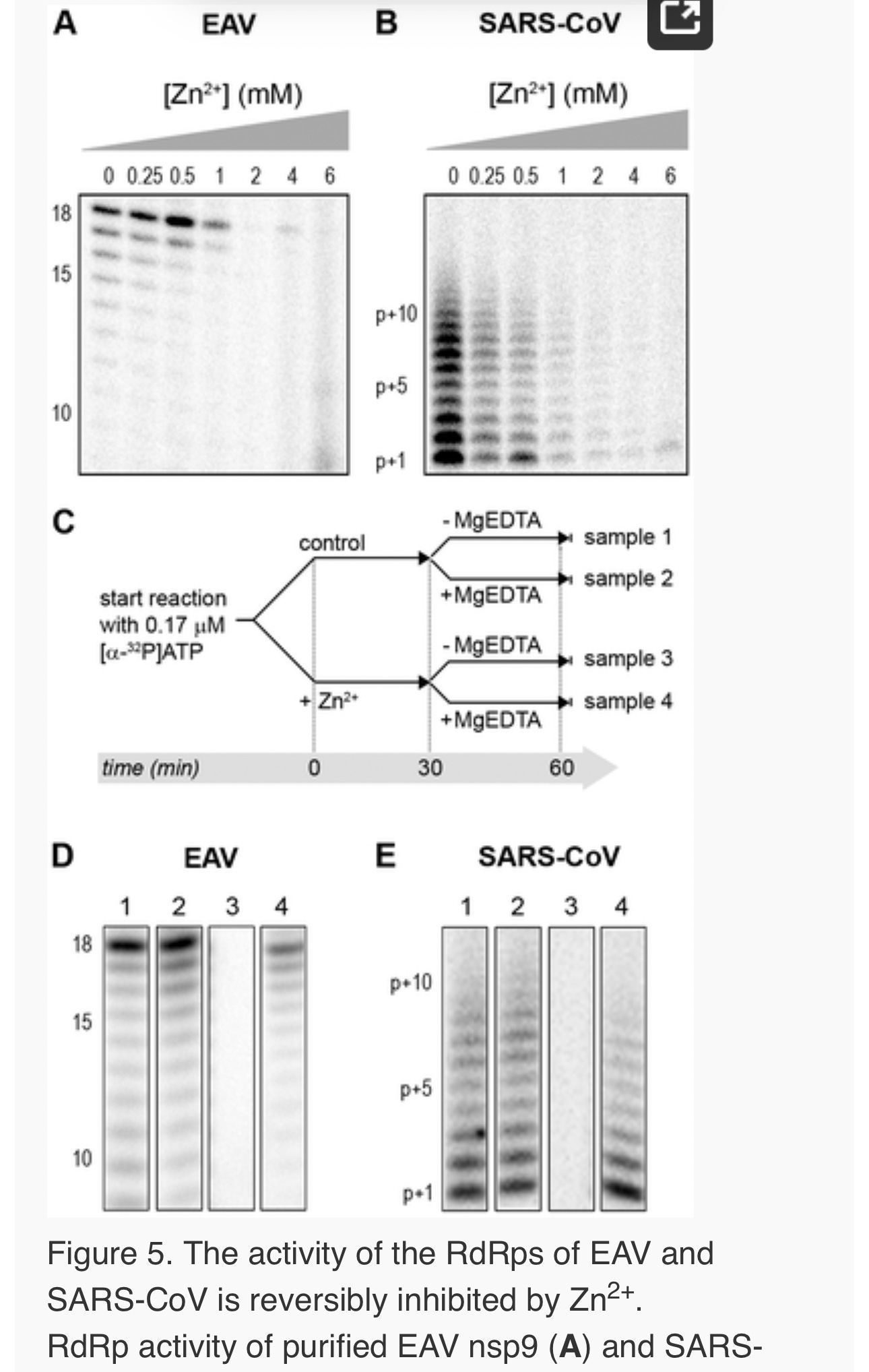
Addition of ZnOAc2 to RdRp assays resulted in a strong, dose-dependent inhibition of enzymatic activity for both the EAV and SARS-CoV enzyme (Fig. 5A and B, respectively), similar to what was observed in RTC assays. In fact, compared to other divalent metal ions such Co2+ and Ca2+, which typically bind to amino acid side chains containing oxygen atoms rather than sulfur groups, Zn2+ was the most efficient inhibitor of SARS-CoV nsp12 RdRp activity (Supplemental Fig. S1). To test whether, as in the RTC assay, the RdRp inhibition by zinc ions was reversible, RdRp assays were pre-incubated with 6 mM Zn2+, a concentration that consistently gave >95% inhibition. After 30 min, 8 mM MgEDTA was added to both a control reaction and the reaction inhibited with ZnOAc2, and samples were incubated for another 30 min (Fig. 5C). As shown in Fig. 5D, the inhibition of EAV RdRp activity by Zn2+could be reversed by chelation of Zn2+ (Fig. 5D; compare lanes 3 and 4). The amount of product synthesized was consistently 60±5% of that synthesized in a 60-min control reaction (Fig. 5D; compare lanes 1 and 4), which was within the expected range given the shorter reaction time. The inhibition of the SARS-CoV RdRp was reversible as well. During the 30-min incubation after the addition of MgEDTA, SARS-CoV nsp12 incorporated 40±5% of the label incorporated during a standard 60-min reaction (Fig. 5E). This was slightly lower than the expected yield and may be caused by the elevated Mg2+ concentration, which was shown to be suboptimal for nsp12 activity [27] and results from the release of Mg2+ from MgEDTA upon chelation of Zn2+.
Differential effect of Zn2+ on the initiation and elongation phase of nidovirus RNA synthesis
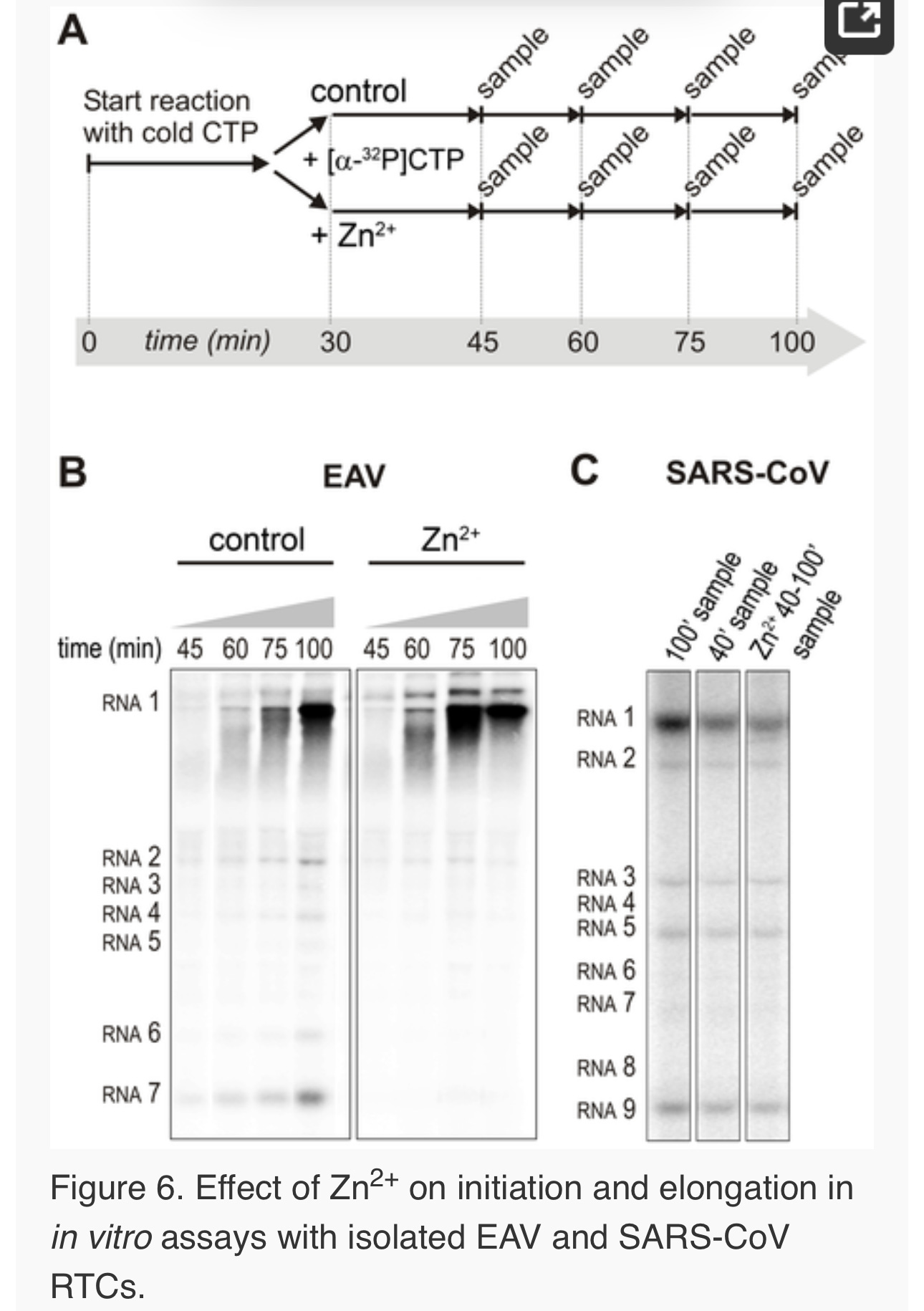
For EAV, close inspection of the RdRp assays revealed a less pronounced effect of Zn2+ on the generation of full-length 18-nt products than on the synthesis of smaller reaction intermediates (Fig. 5A). This suggested that Zn2+ specifically inhibited the initiation step of EAV RNA synthesis. To test this hypothesis, an RTC assay was incubated for 30 min with unlabeled CTP (initiation), after which the reaction was split in two. Then, [α-32P]CTP was added to both tubes (pulse), 500 µM Zn2+ was added to one of the tubes, and samples were taken at different time points during the reaction (Fig. 6A). Fig. 6B shows that in the presence of Zn2+ [α-32P]CMP was predominantly incorporated into nascent RNA molecules that were already past the initiation phase at the moment that Zn2+ was added to the reaction. No new initiation occurred, as was indicated by the smear of short radiolabeled products that progressively shifted up towards the position of full-length genomic RNA. This suggested that Zn2+ does not affect the elongation phase of EAV RNA synthesis and that it specifically inhibits initiation. This also explains the relatively weak signal intensity of the smaller sg mRNA bands (e.g., compare the relative change in signal of RNA2 to RNA7) produced in the presence of Zn2+, since multiple initiation events are required on these short molecules to obtain signal intensities similar to those resulting from a single initiation event on the long genomic RNA, e.g., 16 times more in the case of RNA7. In contrast to EAV, the effect of Zn2+ on RNA synthesis by SARS-CoV RTCs was not limited to initiation, but appeared to impair the elongation phase as well, given that the addition of Zn2+completely blocked further incorporation of [α-32P]CMP when added 40 min after the start of the reaction (Fig. 6C).
In the RdRp assays, the short templates used made it technically impossible to do experiments similar to those performed with complete RTCs. However, we previously noticed that at low concentrations of [α-32P]ATP (∼0.17 µM) SARS-CoV nsp12 RdRp activity was restricted to the addition of only a single nucleotide to the primer [27]. EAV nsp9 mainly produced very short (2–3 nt long) abortive RNA products and only a fraction of full length products, as is common for de novo initiating RdRps [28]. This allowed us to separately study the effect of Zn2+ on initiation and elongation by performing an experiment in which a pulse with a low concentration of [α-32P]ATP was followed by a chase in the presence of 50 µM of unlabeled ATP, which increased processivity and allowed us to study elongation (Fig. 7A and C) as described previously [27]. The results of these experiments were in agreement with those obtained with isolated RTCs. For EAV, with initiation and dinucleotide synthesis completely inhibited by the presence of 6 mM Zn2+ (Supplemental Fig. S2A), the amount of reaction intermediates shorter than 18 nt diminished with time, while products from templates on which the RdRp had already initiated were elongated to full-length 18-nt molecules (Fig. 7B, right panel). This was consistent with the observation that the EAV RdRp remained capable of extending the synthetic dinucleotide ApA to trinucleotides in the presence of Zn2+ (Supplemental Fig. S2B). Likely due to the absence of reinitiation in the reactions shown in Fig. 7B, the low processivity of the EAV RdRp, and the substrate competition between the remaining [α-32P]ATP and the >200 fold excess of unlabeled ATP, the differences between the 5- and 30-min time points were small. In the absence of Zn2+, the RdRp continued to initiate as indicated by the ladder of smaller-sized RNA molecules below the full-length product (Fig. 7B, left panel) and the time course shown in Supplemental Fig. S2A. In contrast, the addition of Zn2+ to a SARS-CoV RdRp reaction also blocked elongation, since extension of the radiolabeled primer as observed in the absence of Zn2+ (Fig. 7D, left panel) no longer occurred (Fig. 7D, right panel).
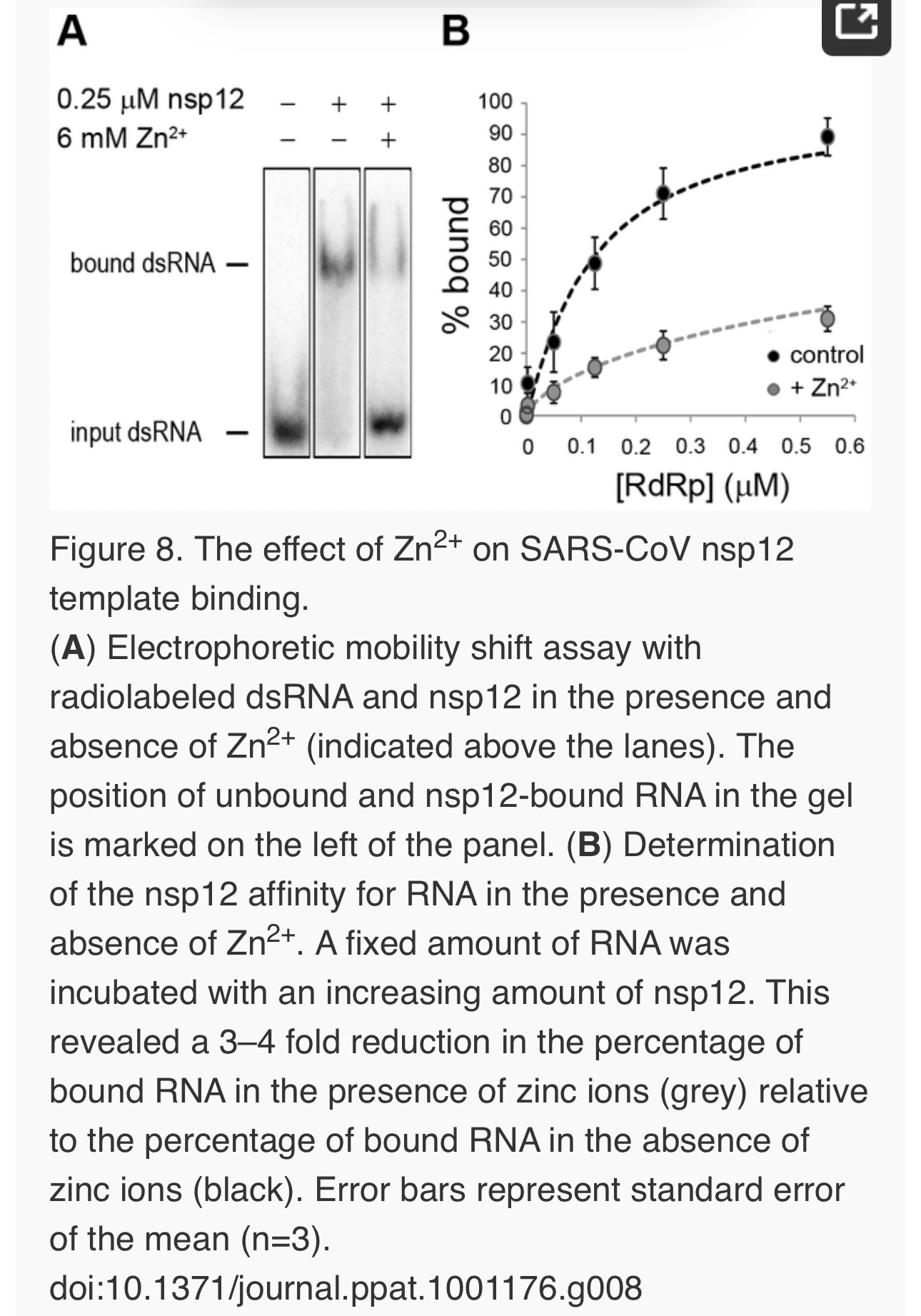
Zinc affects SARS-CoV RdRp template binding
To assess whether Zn2+ affects the interaction of recombinant SARS-CoV nsp12 with the template used in our assays, we performed electromobility shift assays (EMSA) in the presence and absence of Zn2+(Fig. 8A). To measure the binding affinity of the RdRp for the template, we determined the fraction of bound template at various protein concentrations and observed a 3–4 fold reduction in RNA binding when Zn2+ was present in the assay (Fig. 8B). We also assessed whether pre-incubation of the RdRp or RNA with Zn2+ was a requirement for this drop in binding affinity, but found no significant difference with experiments not involving such a preincubation (data not shown).
No binding was observed when a similar RNA binding assay was performed with purified EAV RdRp. Likewise, nsp9 did not bind RNA in pull-down experiments with Talon-beads, His6-tagged nsp9, and radiolabeled EAV genomic RNA or various short RNA templates including polyU, whereas we were able to detect binding of a control protein (SARS-CoV nsp8, which has demonstrated RNA and DNA binding activity [32]) using this assay. It presently remains unclear why we are not able to detect the binding of recombinant EAV nsp9 to an RNA template.
Discussion
Although a variety of compounds have been studied, registered antivirals are currently still lacking for the effective treatment of SARS and other nidovirus-related diseases [33]. RdRps are suitable targets for antiviral drug development as their activity is strictly virus-specific and may be blocked without severely affecting key cellular functions. Several inhibitors developed against the polymerases of e.g. human immunodeficiency virus (HIV) and hepatitis C virus are currently being used in antiviral therapy or clinical trials [34], [35], [36]. Therefore, advancing our molecular knowledge of nidovirus RdRps and the larger enzyme complexes that they are part of, and utilizing the potential of recently developed in vitro RdRp assays [25], [26], [27], [28] could ultimately aid in the development of effective antiviral strategies.
Zinc ions and zinc-ionophores, such as PT and PDTC, have previously been described as potent inhibitors of various RNA viruses. We therefore investigated whether PT-stimulated import of zinc ions into cells also inhibited the replication of nidoviruses in cell culture. Using GFP-expressing EAV and SARS-CoV [29], [30], we found that the combination of 2 µM PT and 2 µM Zn2+ efficiently inhibited their replication, while not causing detectable cytoxicity (Fig. 1). Inhibition of replication by PT and Zn2+ at similar concentrations (2–10 µM) was previously observed for several picornaviruses such as rhinoviruses, foot-and-mouth disease virus, coxsackievirus, and mengovirus [6], [7], [8], [9], [10], [11].
The inhibitory effect of Zn2+ on the replication of picornaviruses appeared to be due to interference with viral polyprotein processing. In infections with the coronavirus mouse hepatitis virus (MHV), Zn2+ also interfered with some of the replicase polyproteins cleavages [24], albeit at a much higher concentration (100 µM Zn2+) than used in our studies. Since impaired replicase processing will indirectly affect viral RNA synthesis in the infected cell, we used two recently developed in vitro assays to investigate whether Zn2+also affects nidovirus RNA synthesis directly. Our in vitro studies revealed a strong inhibitory effect of zinc ions on the RNA-synthesizing activity of isolated EAV and SARS-CoV RTCs. Assays with recombinant enzymes subsequently demonstrated that this was likely due to direct inhibition of RdRp function. The inhibitory effect could be reversed by chelating the zinc ions, which provides an interesting experimental (on/off) approach to study nidovirus RNA synthesis. Addition of Zn2+ following initiation of EAV RNA synthesis had little or no effect on NTP incorporation in molecules whose synthesis had already been initiated in the absence of Zn2+ (Fig. 6 and 7), indicating that Zn2+ does not affect elongation and does not increase the termination frequency, as was previously found for Mn2+ [25]. Therefore, Zn2+ appears to be a specific inhibitor of the initiation phase of EAV RNA synthesis. In contrast, Zn2+inhibited SARS-CoV RdRp activity also during the elongation phase of RNA synthesis, probably by directly affecting template binding (Fig. 8). In coronaviruses, zinc ions thus appear to inhibit both the proper proteolytic processing of replicase polyproteins [23], [24] and RdRp activity (this study). Contrary to the RTC assays, millimolar instead of micromolar concentrations of ZnOAc2 were required for a nearly complete inhibition of nucleotide incorporation in RdRp assays.
It has been well established that DNA and RNA polymerases use a triad of conserved aspartate residues in motifs A and C to bind divalent metal ions like Mg2+, which subsequently coordinate incoming nucleotides during the polymerization reaction [37], [38]. Mg2+ is also the divalent metal ion that is required for the in vitro activity of isolated SARS-CoV and EAV RTCs and recombinant RdRps [25], [26], [27], [28], although de novo initiation of EAV nsp9 is primarily Mn2+-dependent. Zn2+ could not substitute for Mg2+ or Mn2+ as cofactor as it was incapable of supporting the polymerase activity of nidovirus RTCs and RdRps in the absence of Mg2+ (data not shown), as was also reported for the poliovirus RdRp [39]. Moreover, inhibition of nidovirus RdRp activity by Zn2+ was even observed at low concentrations and in the presence of a more than 25-fold excess of Mg2+, suggesting that either the affinity of the active site for Zn2+ is much higher or that Zn2+ does not compete for Mg2+-binding and binds to another zinc(-specific) binding site in the protein.
Specific protein domains or pockets that contain zinc ions may be involved in protein-protein interactions, protein-RNA/DNA interactions, or conformational changes in enzyme structures. Zinc-binding domains commonly consist of at least three conserved cysteine and/or histidine residues within a stretch of ∼10–30 amino acids, such as in zinc-finger motifs and metalloproteases [2], [40], [41]. However, in RdRps there are only few precedents for the presence of zinc-binding pockets, such as those identified in the crystal structure of the Dengue RdRp [42]. Sequence analysis of the EAV nsp9 amino acid sequence revealed that it lacks patches rich in conserved cysteines and/or histidines. In contrast, inspection of the SARS-CoV nsp12 amino acid sequence revealed two such patches, namely H295-C301-C306-H309-C310 and C799-H810-C813-H816. A crystal structure for nsp12 is presently unavailable, but a predicted structure that represents the C-terminal two-thirds of the enzyme has been published [31]. Interestingly, in this model, C799, H810, C813 and H816 are in a spatial arrangement resembling that of the Zn2+ coordinating residues in the Zn2 zinc-binding pocket found in motif E of the Dengue virus RdRp (see Supplemental Fig. S3). Clearly, an in-depth analysis of nidovirus RdRps, e.g. through structural analysis and subsequent mutational studies targeting aforementioned cysteines and histidines, is required to provide further insight into and a structural basis for the Zn2+-induced inhibitory effects on RdRp activity documented in this study. Such studies may, however, be complicated when Zn2+ binding proves to be very transient in nature and not detectable with currently available methods.
In summary, the combination of zinc ions and the zinc-ionophore PT efficiently inhibits nidovirus replication in cell culture. This provides an interesting basis for further studies into the use of zinc-ionophores as antiviral compounds, although systemic effects have to be considered [43], [44] and a water-soluble zinc-ionophore may be better suited, given the apparent lack of systemic toxicity of such a compound at concentrations that were effective against tumors in a mouse xenograft model [45]. In vitro, the reversible inhibition of the RdRp by Zn2+ has also provided us with a convenient research tool to gain more insight into the molecular details of (nido)viral RNA synthesis, and revealed novel mechanistic differences between the RdRps of SARS-CoV and EAV.
Materials and Methods
Cells and viruses
Vero-E6 cells were cultured and infected with SARS-CoV (strain Frankfurt-1; accession nr. AY291315) or SARS-CoV-GFP as described previously [46]. All procedures involving live SARS-CoV were performed in the biosafety level 3 facility at Leiden University Medical Center. BHK-21 or Vero-E6 cells were cultured and infected with EAV (Bucyrus strain; accession nr. NC_002532) or EAV-GFP [29] as described elsewhere [25].
Effect of zinc ions on nidovirus replication in cell culture
One day prior to infection, Vero-E6 cells were seeded in transparent or black (low fluorescence) 96-well clusters at 10,000 cells per well. The next day, cells were infected with SARS-CoV-GFP or EAV-GFP with an m.o.i. of 4, and 1 h p.i. the inoculum was removed and 100 µl of medium containing 2% fetal calf serum (FCS) was added to each well. In some experiments 0–32 µM of pyrithione (Sigma) was added in addition to 0–2 µM ZnOAc2. Infected cells were fixed at 17 h p.i. by aspirating the medium and adding 3% paraformaldehyde in PBS. After washing with PBS, GFP expression was quantified by measuring fluorescence with a LB940 Mithras plate reader (Berthold) at 485 nm. To determine toxicity of ZnOAc2and PT, cells were exposed to 0–32 µM PT and 0–8 µM ZnOAc2. After 18 h incubation, cell viability was determined with the Cell Titer 96 AQ MTS assay (Promega). EC50 and CC50 values were calculated with Graphpad Prism 5 using the nonlinear regression model.
RNA templates and oligonucleotides
RNA oligonucleotides SAV557R (5′-GCUAUGUGAGAUUAAGUUAU-3′), SAV481R (5′-UUUUUUUUUUAUAACUUAAUCUCACAUAGC-3′) and poly(U)18 (5′-UUUUUUUUUUUUUUUUUU-3′) were purchased from Eurogentec, purified from 7 M Urea/15% PAGE gels and desalted through NAP-10 columns (GE healthcare). To anneal the RNA duplex SAV557R/SAV481R, oligonucleotides were mixed at equimolar ratios in annealing buffer (20 mM Tris-HCl pH 8.0, 50 mM NaCl and 5 mM EDTA), denatured by heating to 90°C and allowed to slowly cool to room temperature after which they were purified from 15% non-denaturing PAGE gels.
In vitro viral RNA synthesis assay with isolated RTCs
SARS-CoV and EAV RTCs were isolated from infected cells and assayed for activity in vitro as described previously [25], [26]. To assess the effect of Zn2+, 1 µl of a ZnOAc2 stock solution was added to standard 28-µl reactions, resulting in final Zn2+ concentrations of 10–500 µM. When Zn2+ had to be chelated in the course of the reaction, magnesium-saturated EDTA (MgEDTA) was added to a final concentration of 1 mM. After RNA isolation, the 32P-labeled reaction products were separated on denaturing 1% (SARS-CoV) or 1.5% (EAV) agarose formaldehyde gels. The incorporation of [α-32P]CMP into viral RNA was quantified by phosphorimaging of the dried gels using a Typhoon scanner (GE Healthcare) and the ImageQuant TL 7 software (GE Healthcare).
Expression and purification of nidovirus RdRps
SARS-CoV nsp12 and EAV nsp9 were purified essentially as described elsewhere [27], [28], but with modifications for nsp9. In short, E. coli BL21(DE3) with plasmid pDEST14-nsp9-CH was grown in auto-induction medium ZYM-5052 [47] for 6 hours at 37°C and a further 16 hours at 20°C. After lysis in buffer A (20 mM HEPES pH 7.4, 200 mM NaCl, 20 mM imidazole, and 0.05% Tween-20) the supernatant was applied to a HisTrap column (GE Healthcare). Elution was performed with a gradient of 20–250 mM imidazole in buffer A. The nsp9-containing fraction was further purified by gel filtration in 20 mM HEPES, 300 mM NaCl and 0.1% Tween-20 on a Superdex 200 column (GE Healthcare). The fractions containing nsp9-CH were pooled, dialyzed against 1000 volumes of buffer B (20 mM HEPES, 100 mM NaCl, 1 mM DTT and 50% glycerol) and stored at −20°C. RdRps with a D618A (SARS-CoV) or D445A (EAV) mutation were obtained by site-directed mutagenesis of the wild-type (wt) plasmid pDEST14-nsp9-CH [28] with oligonucleotides 5′-TACTGCCTTGAAACAGCCCTGGAGAGTTGTGAT-3′ and 5′-ATCACAACTCTCCAGGGCTGTTTCAAGGCAGTA-3′, and plasmid pASK3-Ub-nsp12-CHis6 with oligonucleotides 5′-CCTTATGGGTTGGGCTTATCCAAAATGTG-3′ and 5′-CACATTTTGGATAAGCCCAACCCATAAGGA-3′, as described elsewhere [27]. Mutant proteins were purified parallel to the wt enzymes.
RdRp assays with purified enzymes
Standard reaction conditions for the RdRp assay with 0.1 µM of purified SARS-CoV nsp12 are described elsewhere [27]. To study the effect of Zn2+ in this assay, 0.5 µl of a dilution series of 0–80 mM ZnOAc2 was added to the 5 µl reaction mixture, yielding final Zn2+concentrations of 0–8 mM. The EAV RdRp assay contained 1 µM nsp9, 1 µM RNA template poly(U)18, 0.17 µM [α-32P]ATP (0.5 µCi/µl; Perkin-Elmer), 50 µM ATP, 20 mM Tris-HCl (pH 8.0), 10 mM NaCl, 10 mM KCl, 1 mM MnCl2, 4 mM MgOAc2, 5% glycerol, 0.1% Triton-X100, 1 mM DTT and 0.5 units RNaseOUT. ZnOAc2 was added to the reaction to give a final concentration of 0–6 mM. To chelate Zn2+ during reactions, MgEDTA was added to a final concentration of 8 mM. Reactions were terminated after 1 hour and analyzed as described [27].
SARS-CoV nsp12 electrophoretic mobility shift assay
SARS-CoV RdRp was incubated with 0.2 nM 5′ 32P-labeled SAV557R/SAV481R RNA duplex, for 10 minutes at 30°C either in presence or absence of 6 mM ZnOAc2. Reactions were analyzed as described previously [27].
Comments
About the Authors
Aartjan J. W. te Velthuis
AFFILIATION Molecular Virology Laboratory, Department of Medical Microbiology, Center of Infectious Diseases, Leiden University Medical Center, Leiden, The Netherlands
Sjoerd H. E. van den Worm
AFFILIATION Molecular Virology Laboratory, Department of Medical Microbiology, Center of Infectious Diseases, Leiden University Medical Center, Leiden, The Netherlands
Amy C. Sims
AFFILIATION Departments of Epidemiology and Microbiology and Immunology, University of North Carolina at Chapel Hill, Chapel Hill, North Carolina, United States of America
Ralph S. Baric
AFFILIATION Departments of Epidemiology and Microbiology and Immunology, University of North Carolina at Chapel Hill, Chapel Hill, North Carolina, United States of America
Eric J. Snijder
* E-mail: e.j.snijder@lumc.nl (ES); m.j.van_hemert@lumc.nl (MJvH)
AFFILIATION Molecular Virology Laboratory, Department of Medical Microbiology, Center of Infectious Diseases, Leiden University Medical Center, Leiden, The Netherlands
Martijn J. van Hemert
* E-mail: e.j.snijder@lumc.nl (ES); m.j.van_hemert@lumc.nl (MJvH)
AFFILIATION Molecular Virology Laboratory, Department of Medical Microbiology, Center of Infectious Diseases, Leiden University Medical Center, Leiden, The Netherlands
Competing Interests
The authors have declared that no competing interests exist.
Author Contributions
Conceived and designed the experiments: AJWtV SHEvdW EJS MJvH. Performed the experiments: AJWtV SHEvdW MJvH. Analyzed the data: AJWtV SHEvdW ACS RSB EJS MJvH. Contributed reagents/materials/analysis tools: ACS RSB. Wrote the paper: AJWtV EJS MJvH.
Metrics
Media Coverage
SUMMARY
NEWS
BLOGS
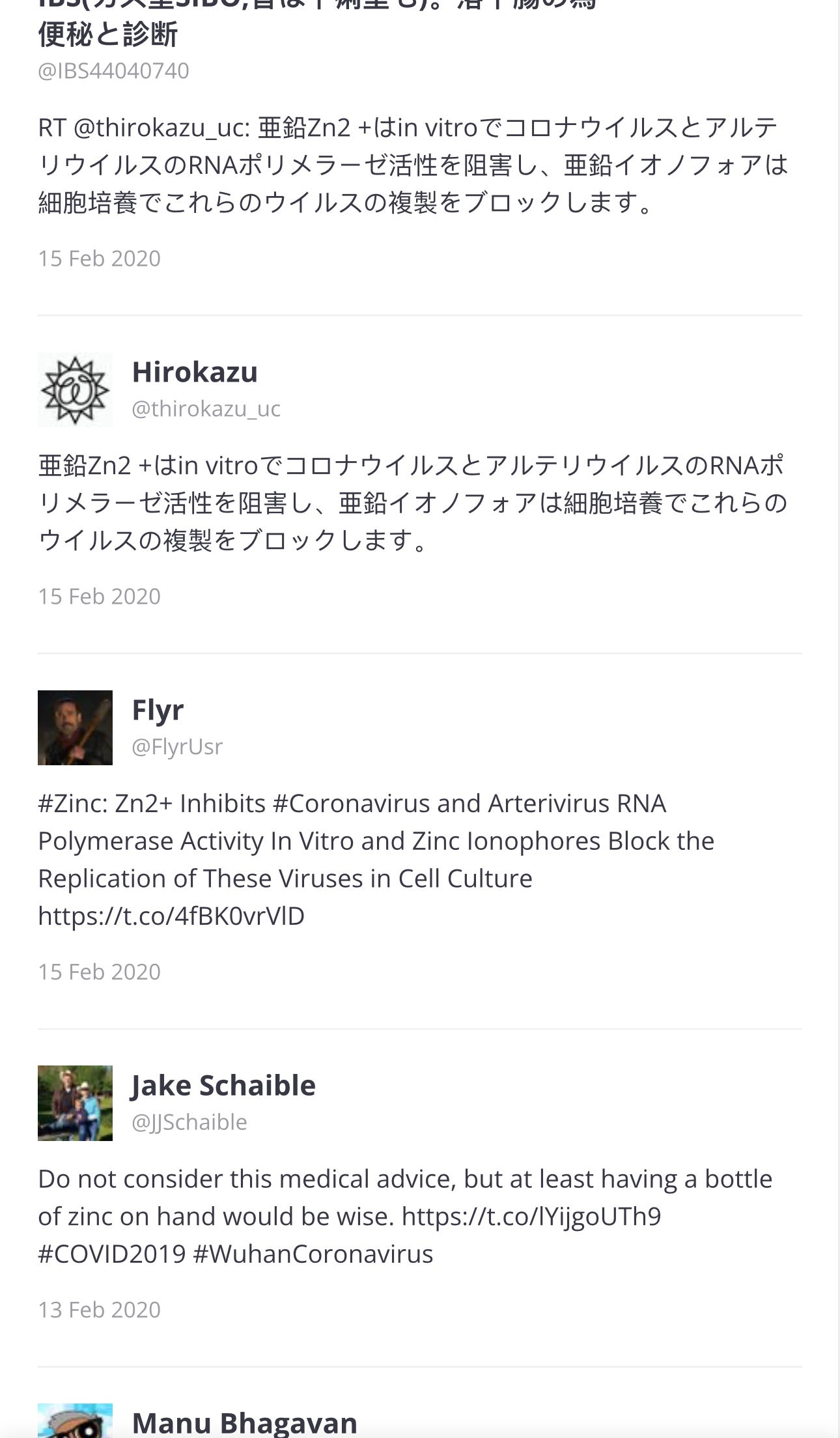
I clicked on the article above, … “Information I GUARANTEE You've Never Heard“ and here is where the link goes:
TWITTER/X
… ⬆️ And all of these tweets are just Page 1 of 69 pages. I will go back to Page 1, to see the first tweets.
Page 1
The paper was published in 2010 and the first three tweets were in 2017 and 2020:
Page 68:
⬆️ Call out to @CDC, Scott GottliebMD
… and for the last four years, Ron Reese and others have been trying to make this connection known.
Now we know. And we have many more questions.
WHAT I REALLY THINK
We can look at every tweet, each deleted blog, and each manipulated headline. We can know exactly who was tagged with this article, and when.
Someone's head is going to roll. Or not. Is there going to be a Nuremberg 2.0? Or nothing?
We need to think about not only what should happen to the evil ones who withheld treatments, but devise a mechanism to stop it from happening again.
And get hydroxychloroquine and zinc to everyone. Heck, the paper even said that zonc ionophore plus zinc was used to treat a tumor.
And what else have they been hiding?
_______________________________
Please leave a comment below or email me at therebelpatiebt@substack.com
Thank you for reading my writings.