Trump's Larry Ellison Promotes AI-created mRNA "Vaccine" for Cancer
It was the “Breakthrough of the Year” in 2013
On Day #2 of President’s Trump’s second term, what’s (all of a sudden) on the menu?
Using AI for the “early detection of cancer”.
They want to use mRNA to create a “cancer vaccine” that is specific to your own body, but more than that, specific to your specific type of cancer. The And it looks like Oracle CEO Larry Ellison, the “Father of the Vaccine” is doubling down on it while simultaneously pushing the technocratic AI Agenda, while Trump says it will create,
“The first data center under the initiative will be built in Texas, with additional expansions planned for other states.”
Source: https://www.newsweek.com/donald-trump-mrna-vaccine-cure-cancer-ai-2018701
This talk was part of Trump’s announcement of a $500 billion project called ‘Stargate’. And they think we think that every individual can combat cancer with another mRNA jab.
mRNA Cancer Vax: It was the “Breakthrough of the Year” in 2013
Source: https://pmc.ncbi.nlm.nih.gov/articles/PMC10447885/
Abstract
Messenger ribonucleic acid (mRNA) vaccines are a relatively new class of vaccines that have shown great promise in the immunotherapy of a wide variety of infectious diseases and cancer. In the past 2 years, SARS‐CoV‐2 mRNA vaccines have contributed tremendously against SARS‐CoV2, which has prompted the arrival of the mRNA vaccine research boom, especially in the research of cancer vaccines. Compared with conventional cancer vaccines, mRNA vaccines have significant advantages, including efficient production of protective immune responses, relatively low side effects and lower cost of acquisition. In this review, we elaborated on the development of cancer vaccines and mRNA cancer vaccines, as well as the potential biological mechanisms of mRNA cancer vaccines and the latest progress in various tumour treatments, and discussed the challenges and future directions for the field.
Keywords: cancer, cancer vaccine, immunology, immunotherapy, mRNA vaccine
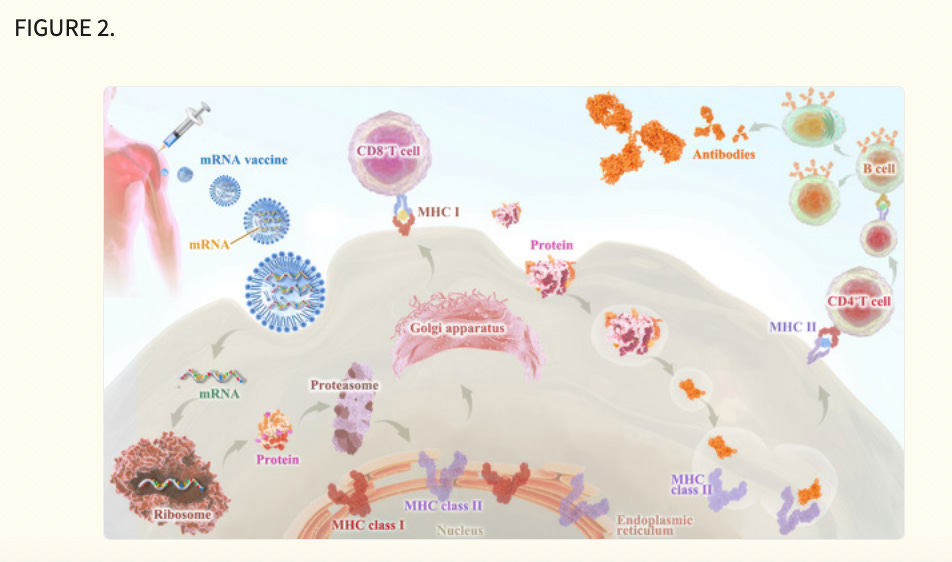
Messenger ribonucleic acid vaccines deliver tumour antigens to antigen‐presenting cells (APCs) for the presentation to immune cells (CD8+ T, CD4+ T) via major histocompatibility complex classes I and II.
Innate immune activity can also be induced through APC‐expressed pattern recognition receptors.
Antigen receptors (chimeric antigen receptors and T cell receptors) can be introduced into lymphocytes.
Expressions of immunomodulatory proteins in different cell types, such as other monoclonal antibody formats, chemokine receptors, costimulatory ligands and Toll‐like receptors, etc are illustrated.
1. INTRODUCTION
As one of the most dreaded diseases in human beings, the emergence of immunotherapy has revolutionized the management of multiple cancer types and shed light on cancer patients. Cancer immunotherapy was determined as ‘breakthrough of the year’ in 2013 due to the successful translation from fundamental research into clinical treatments, as well as the simple yet elegant approach of it. 1 The role of cancer immunotherapy was predominantly underrated in the 20th century on account of the lack of a known mechanism in the field, meanwhile, the routes to develop appropriate clinical schemes are full of twists and turns.
William B. Coley, an American surgeon who was universally acknowledged as the pioneer of immunotherapy, first ventured to manipulate the immune system to tackle patients with inoperable cancer in the late 19th century. 2 , 3 By injecting a mixture of live and inactivated bacteria (called ‘Coley's toxin’) such as Streptococcus pyogenes and Serratia marcescens, Coley found induction of intensive immune response in cancer patients, 4 leading to remission of tumour progression. This trial was deemed the first documented anti‐cancer immunotherapy intervention in history. 5 However, due to the insufficient knowledge of complex humoral immune system and notable advances in traditional treatments at that time, the next revolutionary wave in cancer immunotherapy came late in the 21st century until the consensus was made, 6 when people realized that boosting innate defences to get rid of malignant cells is a monumental milestone for cancer treatment. In recent years, cancer vaccines have attracted tremendous attention due to the striking signs of progress in the field. 7
The cancer vaccine was first developed in 1988. Mitchell et al. immunized melanoma patients with allogeneic melanoma lysate, and successfully induced anti‐melanoma immune response in hundreds of patients. 8 Subsequently, thanks to the discovery of tumour antigens (TAs)—antigenic substances overexpressed in the tumour tissue that regulate tumour initiation, progression and metastasis, scientists are enabling more chances to target tumour cells with the potential candidates for use in cancer therapy. 9 , 10 , 11 , 12 , 13 , 14 In the past 20 years, several methods of antigen delivery have been presented, 15 some of which have shown strong anti‐tumour immune responses and clinical responses in cancer patients.
Technically, the development of tumour vaccines is largely similar to vaccines for infectious diseases, 16 including whole‐cell vaccines, DNA and messenger ribonucleic acid (mRNA) vaccines, antigen vaccines and dendritic cell (DC) vaccines. 17 So far, hundreds of clinical trials have been conducted and demonstrated the promise and challenges posed by therapeutic vaccines (Table 1). Unlike traditional surgery, chemotherapy or radiotherapy, therapeutic cancer vaccines are considered to specifically activate the immune system and target tumour cells, 18 , 19 , 20 leading to higher response rates and better quality of life. Collectively, cancer vaccines are conducive to blocking tumour growth, recurrence or metastasis and recent progress in immuno‐oncology research has exploited an unprecedented avenue for the emergence of vaccine strategies. In recent years, mRNA vaccines have progressed rapidly in the field of tumour biotherapy as an important type of tumour vaccine due to the advantages of low toxicity, fast production and a wide variety of encoded antigens. Specifically, exogenous SAMs encoding tumour‐specific antigens are introduced into somatic cells to induce immune responses by synthesizing antigens and thereby achieving specific killing of tumour cells. The promise and advances of mRNA vaccines in tumour biotherapy have been well established in recent researches. This review summarizes and discusses the advantages and limitations of mRNA vaccines in detail.
2. CANCER VACCINE: STARTING FROM THE BASICS
2.1. Fundamentals of the cancer vaccine
The immune system is a sophisticated network of cells and proteins. Technically, it offers the body protection, so‐called immunity, which is achieved through the presence of antibodies or cell‐mediated immunity to diseases in an individual's immune system, against disease. 21 Among multiple mechanisms of immune response, vaccines are vital to boost the body's immune system, such as by stimulating the production of antibodies against foreign pathogens. Over the past decades, tumour antigen has been extensively exploited and proved to be of great significance in cancer immunotherapy, largely promoting the discovery of cancer vaccines, and eliciting long‐lasting and effective immune responses. 22 In general, cancer vaccines can be classified based on their applications and functions. Classical cancer preventive vaccines target the viruses that can cause certain cancers, 23 whereas therapeutic vaccines can stimulate immune responses against existing tumours. Optimally speaking, 15% of cancer cases could be blocked by fighting infections and it turned out that infection is considered a causative factor in an estimated one in four cancers. 24 Theoretically, it is easier, cheaper and more effective to vaccinate against viruses that lead to cancer than it is to treat cancer with a cancer therapeutic vaccine. However, due to poor immunogenicity and limited safety, only two preventive cancer vaccines have successfully passed clinical trials and have been approved by the U.S. food and drug administration (FDA). 25 , 26
2.2. Vaccines for cancer prevention‐targeting oncogenic virus
Oncoviruses are implicated in approximately 12% of all human cancers. 27 The evidence that chronic infections with hepatitis B virus (HBV) and high‐risk human papillomavirus (HPVs) are important caustic factors for hepatocellular carcinoma and cervical cancer, respectively, 28 , 29 has been underpinned solidly over the past three to four decades. The HBV vaccine is known as the first ‘anti‐cancer’ vaccine, which was first implemented for HBV in 1982, with the development of a highly innovative, novel prophylactic vaccination approach, widespread HBV immunization of neonates has exerted a great impact on the incidence of chronic HBV infection in children 5 years of age. 30
Additionally, HPV is a universal public health problem with high rates of cervical cancer, it was confirmed as a causal agent of cervical cancer by Harald Zur Hausen, 31 a German virologist, who first isolated HPV strains in cervical cancer tumours in the 1980s, this theory led to the completion of the first human trial for the HPV vaccine, named Gardasil in 2006. 32 Since then, two further vaccines have been approved: Cervarixin 2007 and Gardasil 9 in 2014. 33 , 34 As of June 2020, more than 100 countries worldwide have included HPV vaccine in their national immunization programs as part of their regular vaccine schedule. Nonetheless, many oncogenic DNA viruses have been under investigation. 35
2.3. Vaccines for cancer treatment: New hope in the cancer immunotherapy
Unlike prophylactic vaccines which are exploited against viruses, the therapeutic cancer vaccine aims to operate the immune system to mount an attack against cancer cells or tissues in the body. 36 Technically, the precondition for the development of a therapeutic cancer vaccine depends on the presence of the tumor‐associated antigen (TAA) or tumour‐specific antigens (TSAs). 37 To date, most cancer vaccines have targeted TAAs, which are self‐proteins that are abnormally expressed by cancer cells, nevertheless, obstacles have to be overcome before developing vaccines against TAAs. For example, immune cells may recognize TAAs as self‐antigen, remove them from the immune repertoire, leading to failure in immune responses. 38 , 39 TSAs represent antigens that are specific to tumour cells, and have aroused extensive attention due to their specific reaction with immune cells. However, due to uniqueness of neoantigens to each patient and tumour type, further optimisation needs to be conducted for the sake of reducing the cost and complexity of TSA vaccine. 40
Although major challenges in terms of efficacy and safety are still existing, diverse therapeutic vaccination strategies have been under development in the pre‐clinical stage or evaluated in clinical trials. Based on the platforms used in vaccine development, therapeutic vaccines are classified into various major categories. 41 Among those, mRNA vaccines are a promising alternative to conventional vaccine approaches. 17 Up to now, multiple mRNA cancer vaccines have been employed in various clinical trials, leading to the notion that this strategy can be broadly applicable to cancer vaccines. 42
3. mRNA CANCER VACCINES: HISTORY AND RECENT ADVANCES
As aforementioned, cancer and infectious diseases are the two most common challenges humankind is faced with currently, despite multiple in‐progress research and treatment measures, satisfactory results are yet to be achieved. The emergence of new pandemics like COVID‐19, Ebola, Zika, HIV and measles has necessitated the need for finding a new approach or new technique unlike before to overcome the crisis in healthcare and for the betterment of human life. 43 , 44 , 45 , 46 A dormant technique that was considered almost impossible to be implemented in the 19th century was ventured again in the last decade and reinstalled confidence in the form of nucleic acid encoded therapeutics. Recent advances in research have given us deeper knowledge of human genetics and have created an avenue for mRNA as a promising treatment protocol for infectious diseases and cancer. 17 , 47 The human genome contains nearly 21306 genes that code for proteins. 48 The genes in the DNA are transcribed into mRNA inside the nucleus of a cell. The transcribed mRNA is a highly unstable molecule that carries information from genes in the nucleus to ribosomes in the cytoplasm for protein synthesis. 17 So, mRNA is the intermediate of the protein synthesis process in our body. Given that mRNA can be translated into proteins, this principle can be utilized to produce any protein like defective enzymes, hormones, antibodies, cell structure proteins and foreign antigens. 49 As a result, mRNA vaccines are eye‐catching as they are easy to produce, manipulate and deliver into cells. 50 , 51 In comparison, other techniques of genomic/genetic engineering need manipulation of the genome that might lead to undesirable effects such as off targeting. These effects can be minimized by using mRNA vaccines. Of note, in cancer, mRNA is used to induce the immune system to destroy tumour‐associated antigens and growth factors. 52 , 53 , 54 The current COVID‐19 pandemic has necessitated the need for the development of a new vaccine and has accelerated research developments in mRNA therapeutics at a tremendous pace. 55 , 56 , 57 , 58 , 59
Although vaccinology has made outstanding achievements in preventing various diseases, there are still major hurdles to the development of vaccines against cancer, emphasizing the necessity to develop a more effective and functional vaccine platform. 60 However, due to mRNA instability, high innate immunogenicity and in vivo delivery efficiency, the study of mRNA vaccines once lagged behind the research of DNA and protein‐based vaccine development until technological innovations and research investments recognized mRNA as a versatile tool for the development of new innovative therapeutics, especially in the field of vaccinology in the past decades. 42
3.1. History
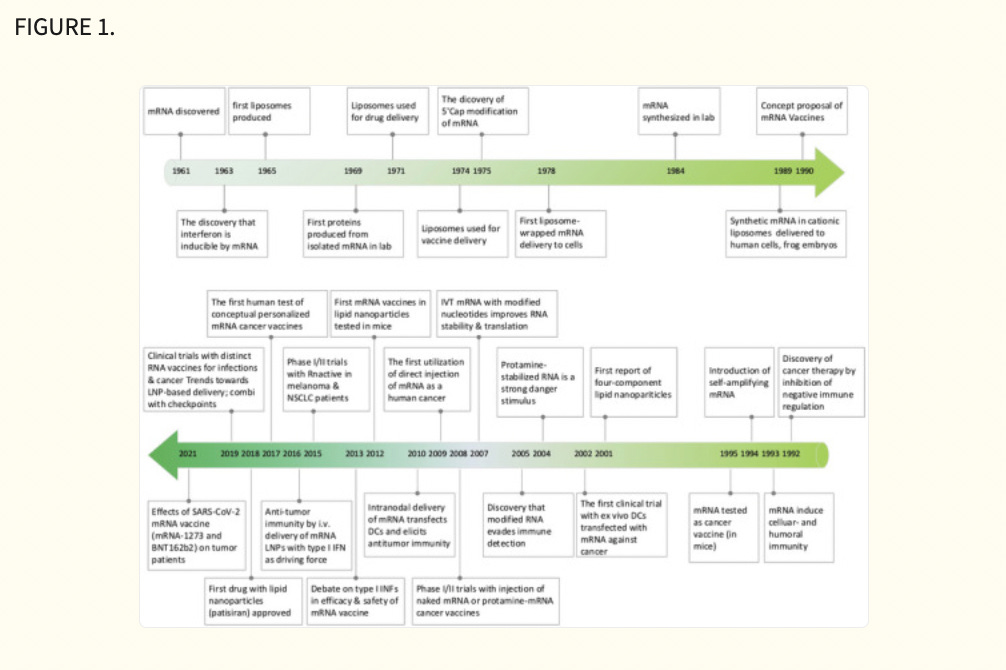
The study of mRNA structure and function has been fascinating since the first human discovery of mRNA in 1961. 61 Notably, the first in vitro translation of isolated mRNA was achieved by humans in 1969, which supported researchers in synthesizing specific proteins in vitro. 62 Also, with the rapid advancement of vaccine delivery systems, researchers developed liposome‐encapsulated mRNA delivery systems in 1978, which made it possible to regulate proteins within target cells. 63 , 64 This was followed by Jirowski et al. (1992) who successfully used vasopressin mRNA to treat diabetes in mice insidiously. Most importantly, in 1990 Wolff et al. used mRNA‐encoded proteins for vaccination in mice for the first time. 65 Jirowski et al. followed Wolff with the successful use of pressin mRNA for the treatment of diabetes in mice. 66 These results widened the scope of mRNA‐based therapeutics. In 1995, in tumour immunotherapy, researchers confirmed the potential of mRNA applications with the first introduction of mRNA‐encoded luciferase and carcinoembryonic antigen vaccines with good results. 67 However, this technology has gradually been clinically validated in recent years with breakthroughs in technical difficulties related to mRNA structural stability and delivery methods. Into the 2000s, mRNA vaccines entered a period of rapid development because of their unique advantages (ease and rapidity of design and detection, inherent immunogenicity, quick preparation and negligible risk of insertional mutagenesis). This was followed by a remarkable achievement in 2005 when nucleoside‐modified RNA was found to be non‐immunogenic, which brought new light to further mRNA vaccine research. 68 Prostate cancer vaccines entered clinical trials in 2015. 69 In 2019, the variable splicing of SAMs for cancer immunotherapy was found by researcheres. 70 Importantly, in 2020 and 2021, the medical value of mRNA vaccines was realized with the approval of two mRNA vaccines against COVID‐19, Comirnaty (BNT162b2) and Spikevax (mRNA‐1273) 71 , 72 (Figure 1). Nowadays, mRNA vaccines are often reported, and in the future, they may become one of the essential technologies for disease prevention and treatment. 19
3.2. Advantages of mRNA cancer vaccines
Although the application of mRNA vaccines in cancer treatment is still nascent, numerous features of in vitro transcribed mRNA have indicated its vaccine potential, additionally, mRNA vaccines have manifested several striking advantages over peptide or DNA vaccines, which could be more effective against a wide range of cancers.
First, the development of RNA‐based vaccines is relatively faster and cheaper than conventional vaccines owing to the high yields of in vitro transcription (IVT) reactions 73 and the advanced industrial setup which revolutionized the manufacture of mRNA and significantly reduces the cost of production to an extent. 74 For example, in a phase 1 clinical trial in 2020, the first volunteer was administrated the COVID‐19 mRNA vaccine 10 weeks after the sequence of the viral genome revealed. 75 As of November 2020, there were already two novel mRNA vaccines awaiting authorization as potential COVID‐19 vaccines, mRNA‐1273 from Moderna and BNT162b2 from a BioNTech/Pfizer partnership. 76 , 77 The faster production capability of mRNA vaccines is of great value to herald rapid control over the spread of various infectious diseases as well as multiple types of cancer.
Second, mRNA vaccines are not manufactured with pathogen particles or inactivated pathogens, the non‐infectious attribute largely decreases the risk of undesired immune responses. 42 Furthermore, compared to DNA cancer vaccines, the administration of mRNA is through a non‐integrating platform which is only required to be present in the cytoplasm other than entering the nucleus of a cell as a DNA vaccine. This feature bypasses the risk of integrating a foreign gene into the host genome and may eliminate the additional cellular (i.e., nuclear) membrane that plasmid DNA needs to cross, consequently, there is no potential risk of infection or insertional mutagenesis. Additionally, mRNA degradation is controlled by physiological cellular processes, thanks to the progress of various modifications and delivery methods, the in vivo mRNA half‐life can be therefore designed to be under regulation, which again largely guaranteed the safety of mRNA vaccine.
Third, early clinical trial results have indicated that mRNA vaccines have generated a reliable immune response and are well‐tolerated by healthy individuals with relatively high efficiency (Figure 2). On the one hand, with the help of IVT, the simple procedure that allows for the template‐directed synthesis of RNA molecules of any sequence from short oligonucleotides to those of several kilobases, 78 mRNA can be produced in a cell‐free environment by not only avoiding the contamination of microbes or the quality and safety issues in the cultured cells production 79 but also largely improve the manufacturing efficacy, accelerating downstream purification and leading to rapid and cost‐effective manufacturing. On the other hand, current achievements in the field of in vivo delivery have successfully formulated mRNA into carrier molecules, for instance, nanoparticles, allowing rapid uptake and expression in the cytoplasm. 80 , 81 , 82 , 83 , 84 In addition to complexing conventional mRNA into stable nanoparticles, a further improvement in RNA vaccination could be gained using self‐amplifying RNA or replicon RNA (RepRNA), which are derived from the genome backbone of an alphavirus in which the genes encoding the viral RNA replication machinery are intact, but the encoding viral structural proteins are replaced with a transgene encoding the vaccine antigen, substantially inducing strong immune responses. Precision, or personalized medicine, as a novel approach to healthcare based on each person's unique genetic makeup has provided a genomic blueprint to determine an individual's unique disease susceptibility and opened a new chapter for disease prevention and treatment. 85 , 86 Due to the usage of TSAs or TAA in mRNA cancer vaccine development, mRNA‐based personalized cancer vaccines have the potential to tailor therapy with the best response and highest safety margin to ensure better patient care. 87
Technically, through next‐generation sequencing, neoepitopes could be identified on a patient's tumour cells, which guides the immune system to distinguish cancer cells from normal cells. Subsequently, the mRNA vaccine could be tailored to fit the specific antigen repertoire of each patient tumour, once injected, it has the potential to direct the patient's cells to express the selected neoepitopes and conduct clearance. In conclusion, these advantages demonstrate inherent high‐efficiency features optimal for cancer therapeutic use. 42
3.3. Classification of mRNA cancer vaccines
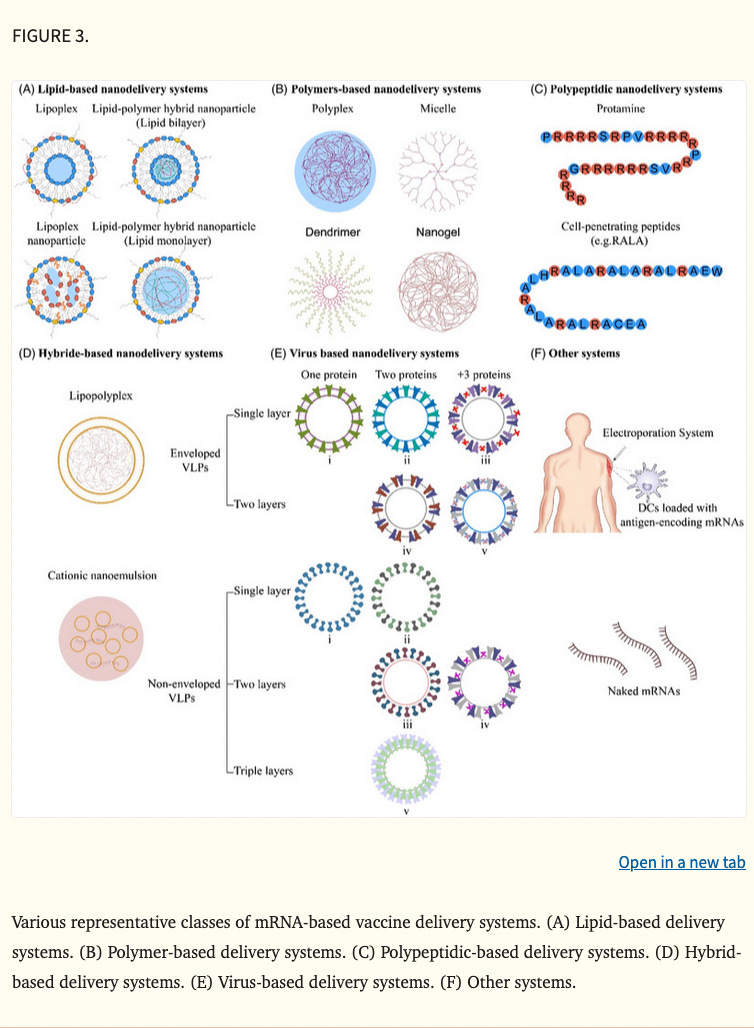
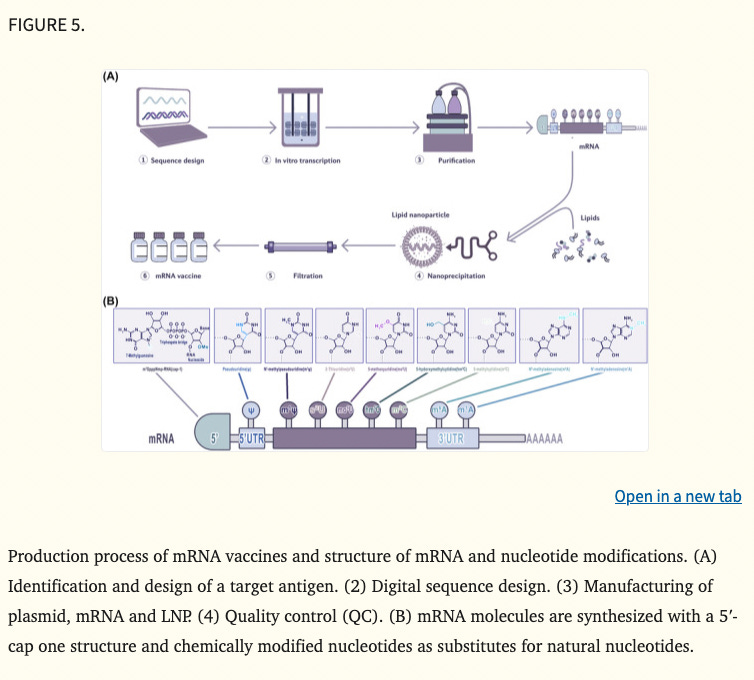
mRNA cancer vaccines are broadly classified into various categories depending on their morphology, size, physical and chemical properties. Some of them have been widely used around the world, such as lipid‐based nanodelivery systems (Figure 3A), 88 polymer‐based nanodelivery systems (Figure 3B), 89 polypeptidic nanodelivery systems (Figure 3C), 90 hybrid‐based nanodelivery systems (Figure 3D), 91 virus‐based nanodelivery systems (Figure 3E) 92 and others (Figure 3F). Based on different vaccine characteristics, some of the well‐known classes are provided in Figure 3.
3.3.1. Based on therapeutic or prophylactic property
The therapeutic vaccines are designed to induce cell‐mediated immunity to eradicate cancer cells, however, the aim of the prophylactics is to product the antibodies. 93 Therapeutic cancer vaccines paid more attention to autoimmune complications, as the autoimmune complication is still an unsolved problem in preventive therapies. Although HPV and HBV vaccines have been approved by FDA and made a good score in cancer prevention, clinical trials of prophylactic mRNA cancer vaccine have not yet been reported. The therapeutic and prophylactic vaccines are not quite distinct from each other, sometimes prophylactic vaccines can also be used as therapeutic vaccines because they are tested effective in the reduction of the risk of clinical relapse. 94
3.3.2. Based on types of mRNA
Based on the RNA structure, there are mainly three types of mRNA cancer vaccines: non‐replicating mRNA (nrRNA) vaccine, self‐amplifying mRNA (SAM) vaccine and trans‐amplifying mRNA vaccine.
The nrRNA vaccine is a synthetic analogue of mature mRNA, whose constructs contain conventional mRNA vaccine sequences, usually including the universal 5ʹ Cap, 5ʹ untranslated regions (UTRs), an open reading frame (ORF), 3ʹUTRs and a 3ʹpoly(A) tail. The simple structure and relatively small size are the main advantages, but correspondingly, this also limits the activity and stability of nrRNA vaccine constructed in vivo, which is the major disadvantage. To enhance the durability of antigen expression, adjuvants can be added to optimise the structure of RNA molecules. TriMix is a kind of new potent adjuvant strategy with high safety, which demonstrated a superior T cell stimulatory capacity and enhancement of DCs’ maturation. 95 , 96 TriMix is developed by Vrije Universiteit Brussel, consisting of SAMs that encode three immune activator proteins—CD70, CD40 ligand (CD40L) and constitutively active TLR4. 97 The application of TriMix naked mRNA in AIDS patients has achieved the desired results and aroused popular concern. 98 For cancer therapy, De Keersmaecker B's clinical studies 99 indicated great anti‐tumour responses in patients with terminal stage of melanoma by using TriMix electroporated together with a DC‐based mRNA vaccination.
The SAM vaccines are produced through gene engineering of positive‐stranded RNA viruses, including alphaviruses, picornaviruses, flaviviruses and so on, whose gene encoding structural proteins are replaced by antigen sequence. 100 Compared with the nrRNA, there is an extra replication component in the construction of SAM vaccine, directing the amplification of intracellular mRNA after delivery to targeted cells. 101 As a consequence, SAM vaccines have a higher level of antigen expression and long‐lasting efficacy with lower dosages, which is their obvious advantage.
Trans‐amplifying mRNA vaccines are a novel type which was designed by Beissert et al. 101 It has a replicase that can amplify the RNAs ‘in trans’, which means two genes act simultaneously on different RNAs. This special structure permits a shorter length of RNA, which could reduce the difficulty of scaled‐up production and manufacturability, however, consequently adding the complexity of delivery and manufacture of two RNA drugs. Although trans‐amplifying mRNA vaccines have not been reported in clinical cancer therapy, this approach has a promising future since it could be further improved by implementing new strategies benefiting from its special structure.
3.3.3. Based on route of administration
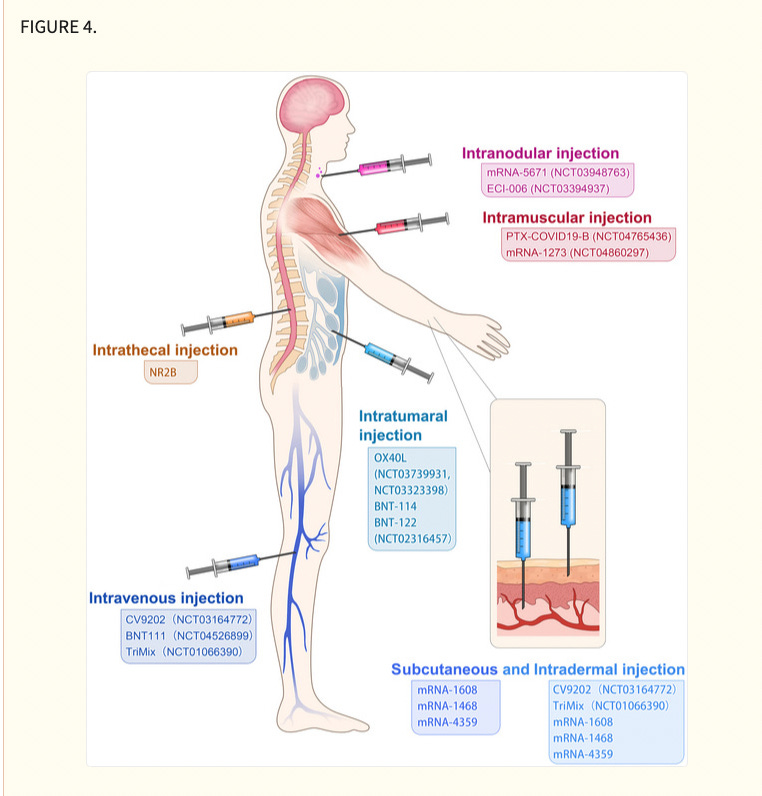
Although systematic evaluation of various administration routes in animal models or patients remains to be studied, different administration routes influence efficacy and immune‐stimulation area of mRNA cancer vaccines. At present, administration strategies of mRNA cancer vaccines include subcutaneous injection, 102 intradermal injection, 103 intranodular injection, 104 intramuscular injection, 105 intravenous injection, 106 intratumoural injection, 107 intrathecal injection 108 and so on (Figure 4), which are primary but economical methods of stimulating immune responses. Local injection is considered a highly immunocompetent method, which augments local vaccine response and triggers a distal immune reaction by lymphatic transport. 103 Intranodal or near‐nodal (into soft tissue) immunizations were usually instructed by surgical exposition of a mouse lymph node. Although intranodal injection has a complicated operation, potent T cell immunity was observed compared with other administration routes (i.d./s.c./n.n.) by Kreiter et al. 104 Tracheal administration, targeting the lung vasculature, is usually used in pulmonary lesions, with mRNA administered as aerosol. The intrathecal injection is applied in brain lesions to help antigen presentation in cells of the central nervous system which are hard to reach. For systemic delivery, mRNA cancer vaccines are commonly administered as nanosized drug formulations and delivered intravenously, mainly targeting liver due to its abundant fenestrated capillaries.
.4. Recent innovations in mRNA vaccine technologies
The basic structure of mRNA includes a 5′ cap, 5′ UTR, coding region, 3′UTR and a poly (A) tail. 109 The 5′UTR or 5′ caps are crucial for producing protein efficiently, these structures may regulate cap‐dependent translation initiation. 110 , 111 The 3′UTR consisting of optimal poly (A) signal is required for the stability of mRNA and augmentation of protein translation. 112 , 113 Besides, codon optimisation is helpful to promote protein production, the abundance and stability of mRNA. 114 , 115 In a word, the stability and translation of mRNA determine the success of RNA vaccine production (Figure 5A). The conventional technologies, such as incorporation of modified nucleosides, optimisation of coding sequences, intranodal delivery of mRNA, ex vivo‐loaded DCs, gene gun and electroporation were a huge breakthrough for mRNA vaccine production, 116 , 117 , 118 whereas these methods are complicated or costly or too hard to be used in human. Therefore, innovative technologies are needed to efficiently produce the mRNA vaccine. Recently, there are three most significant innovations in mRNA vaccine technology 119 : (1) modification of mRNA structural elements, (2) optimisation of mRNA manufacturing platform and (3) development of mRNA delivery system.
…
After all, we know that they are causing cancers with the Covid MRNA jab, so they are poised for MRNA vax 2.0
Lots of oncologists know that we have much more turbo cancer since the Covid jab.
Source: https://x.com/DailyCaller/status/1881837884824858706
The Video
What About God?
We will undoubtedly see people with cancer is all around us. Let’s get back to relying on Him for everything.
We just got back from another urology visit. Ed has high-grade transitional bladder cancer they say requires infusion of chemo into his bladder once a week for six weeks. People get nauseated, and he will pretty much be sick for the entire time.
There are two chemos available, BCG from the tuberculosis virus, and the same chemo that Ed already had gemcitabine (that made him sick). And he swore that he would never go back again for chemo.
Or, he could sit and watch, with cystoscopy every three months; they would just keep taking out whatever they find.
You and I know that God has this, and those tumors are SHRINKING in the Name of Jesus! I just want Ed to know! This is his time to believe, and he has to do it on his own (with our added prayer and intercession).
Thank you for your prayers.
The Response
WHAT I REALLY THINK
Where’s God? WHY ARE WE RELYING ON BIG PHARMA AGAIN?
Oh! Because it’s more $.
LET US PRAY
Thank You, Father,
For all the things You do for us. We are so blessed and grateful for each day, each moment. Keep guiding us, Lord, keep leading us in all the ways we should go.
Help Ed trust in You, help everyone who is sick to place their faith and belief in Your love, light, and powerful healing miracles!
We SPEAK LIFE into our lives, into Ed’s life, and into all of our lives! We PRAISE YOU for Your presence! We THANK YOU for loving us and caring about all the things we care about!
Lord we need You! We find our rest in You! You are the one that guides our hearts!
We ask these things in the Name of Jesus.
Amen.













I don't trust them anymore than I did when I smelled the noxious odor of a rat in early '20
Trump needs to kick Ellison and his mRNA war on mankind to the curb immediately and get back to basics…
There’s no moment to soon for this to occur.