First Synthetic mRNA RSV Jab by MODERNA is Now APPROVED by the FDA: mRNA-1345 (mRESVIA®)
WITHOUT Long-term, genetic, or cancer testing! APPROVED for Age 60 and Up. Includes the Package Insert, Information for Professionals, Caregiver Info, and Cost (the latter per Colorado Law).
On May 31, 2024, the FDA approved the first mRNA jab for Respiratory Syncytial Virus (RSV) by Moderna, who proudly displays the announcement on it website.
Although they failed us during Covid, they're asking us to trust them again, this time with another cold virus. And it's going to be treated with another mRNA jab.
Why Do We Need a mRNA RSV Jab?
Why do we even NEED an RSV jab, when the risk of serious disease from it are so rare to be almost laughable? The ConquerRSV study reports this finding, that RSV is very rare, so how could that be worth the risk of a genetic mRNA jab?
The above study was on “high-risk” for RSV patients.
Out of 35,541 participants, only 20 got sick!
This was defined by cough, fever, and nasal congestion, cough. The rate of sickness was only .056%! How do they KNOW they really had RSV? And remember, there is no “TEST” for RSV, which presents as a common cold. Why get a shot for something that you may not have?
The Moderna Website Press Release:
RSV is a highly contagious seasonal respiratory virus and a leading cause of lower respiratory tract infections and pneumonia that causes a particularly large burden of disease in infants and older adults. Each year in the U.S., approximately 60,000-160,000 older adults are hospitalized and 6,000-10,000 die due to RSV infection.[1]
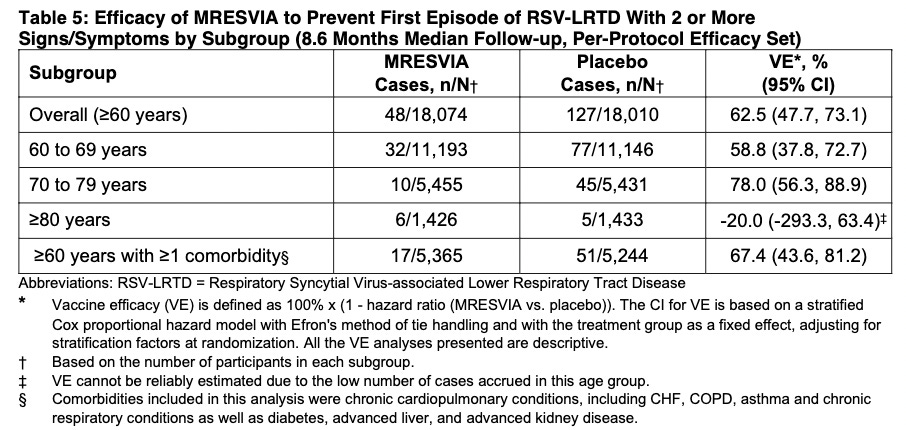
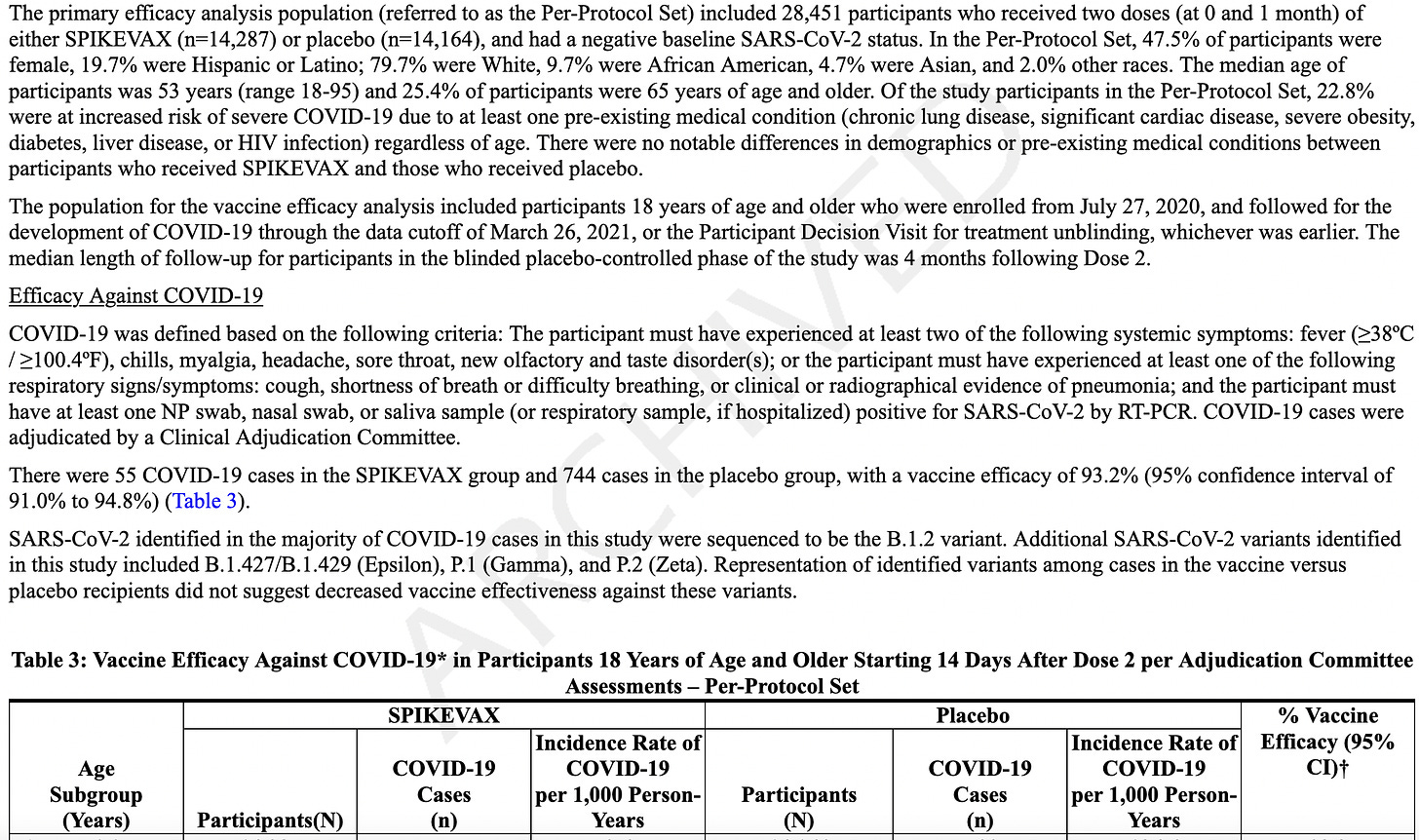
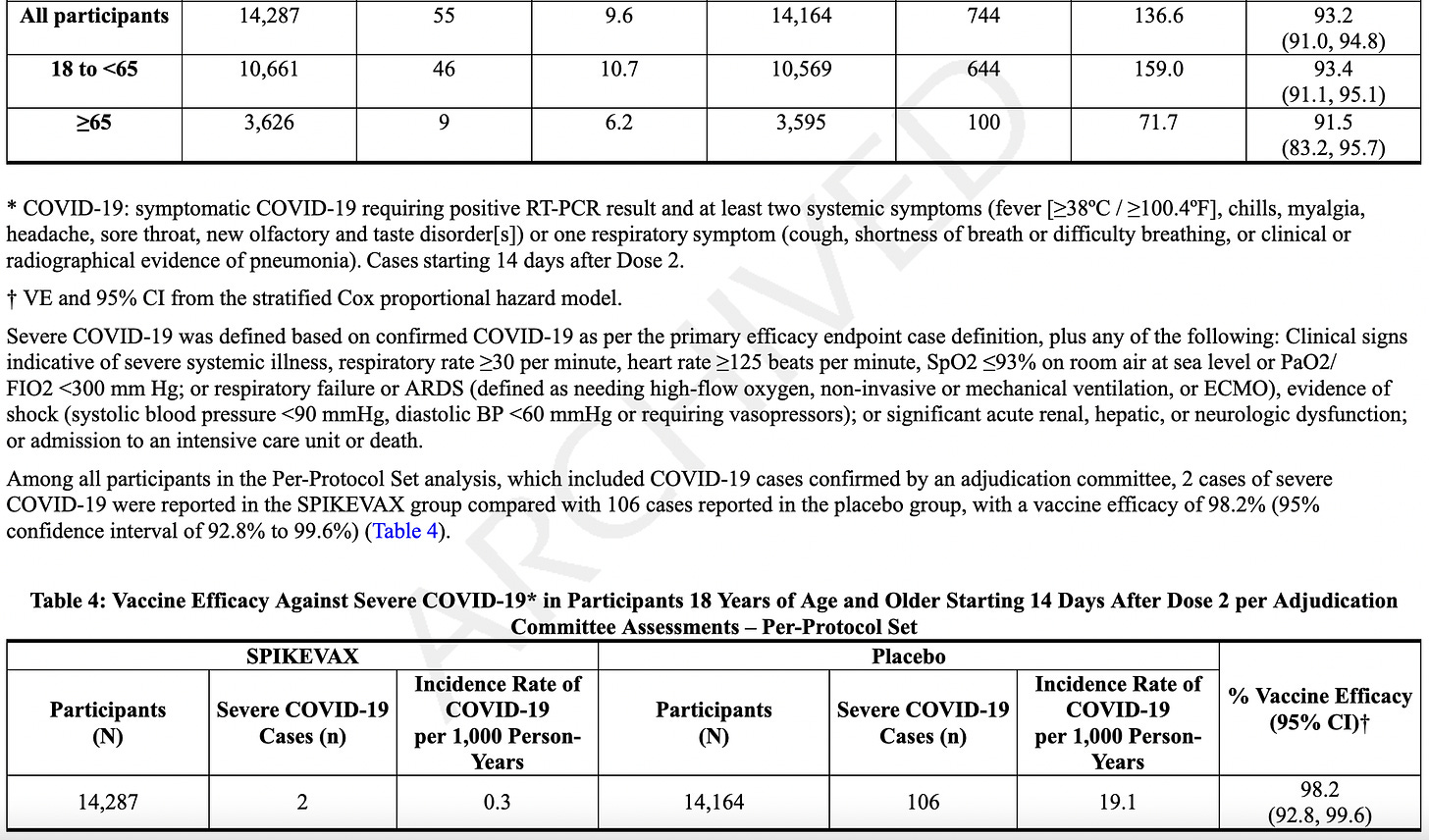
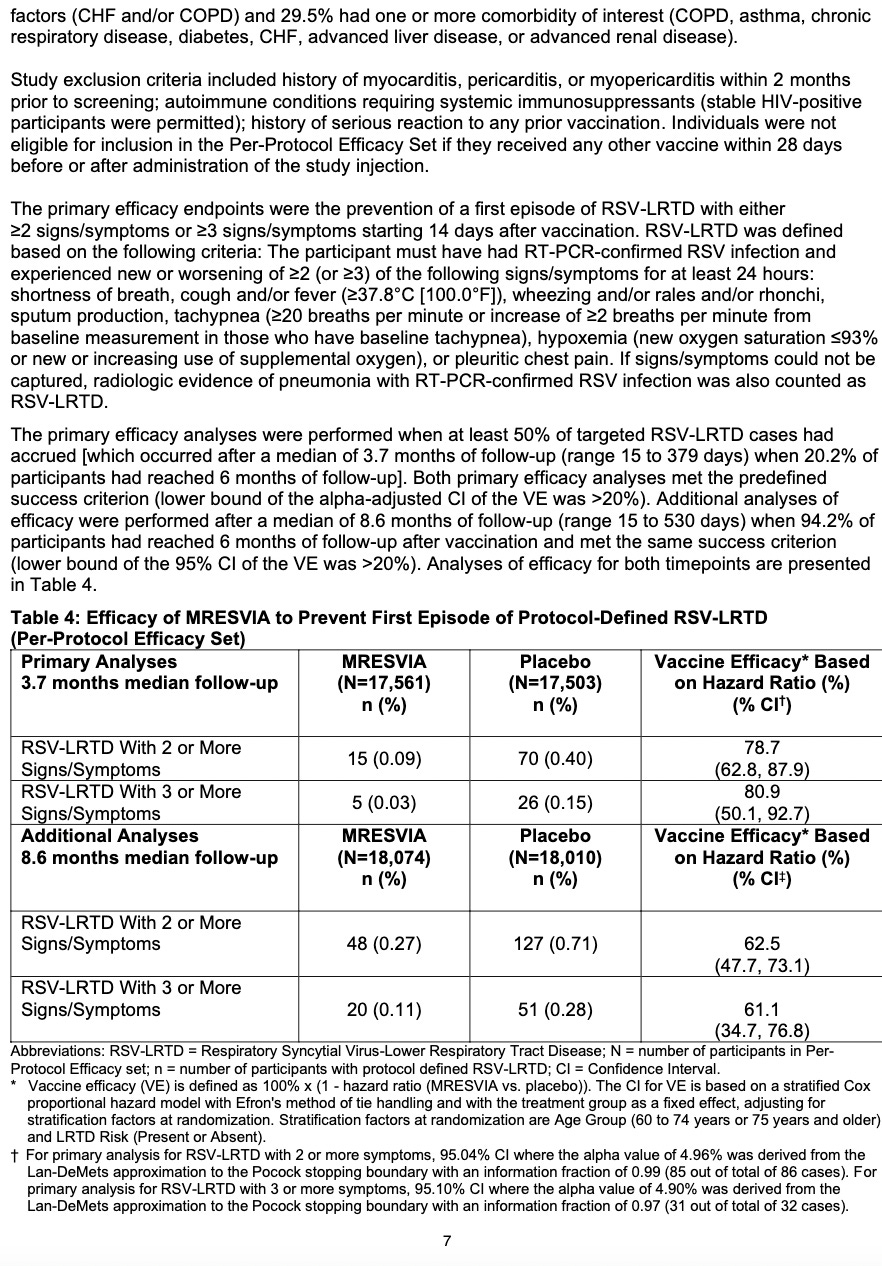
The FDA's approval of mRESVIA is based on positive data from the Phase 3 clinical trial ConquerRSV, a global study conducted in approximately 37,000 adults ages 60 years or older in 22 countries. The primary analysis with 3.7 months of median follow-up found a vaccine efficacy against RSV lower respiratory tract disease (LRTD) of 83.7% (95.88% CI 66.0%, 92.2%). These results were published in The New England Journal of Medicine. A follow-up analysis of the primary endpoint was performed during FDA review, including cases that started before the primary analysis cut-off date but were not confirmed until afterward. The results were consistent with the primary analysis [VE 78.7% (CI 62.9%, 87.8%)] and were included in the U.S. package insert. An additional longer-term analysis showed continued protection against RSV LRTD over 8.6 months median follow-up.
No serious safety concerns were identified in the Phase 3 trial. The most commonly reported solicited adverse reactions were injection site pain, fatigue, headache, myalgia and arthralgia.
Moderna expects to have mRESVIA available for eligible populations in the U.S. by the 2024/2025 respiratory virus season.
Moderna has filed for mRNA-1345 approval with regulators in multiple markets around the world.
About mRESVIA® (Respiratory Syncytial Virus Vaccine)
mRESVIA®is an RSV vaccine that consists of an mRNA sequence encoding a stabilized prefusion F glycoprotein. The F glycoprotein is expressed on the surface of the virus and is required for infection by helping the virus to enter host cells. The prefusion conformation of the F protein is a significant target of potent neutralizing antibodies and is highly conserved across both RSV-A and RSV-B subtypes. The vaccine uses the same lipid nanoparticles (LNPs) as the Moderna COVID-19 vaccines.
About Moderna
Moderna is a leader in the creation of the field of mRNA medicine. Through the advancement of mRNA technology, Moderna is reimagining how medicines are made and transforming how we treat and prevent disease for everyone. By working at the intersection of science, technology and health for more than a decade, the company has developed medicines at unprecedented speed and efficiency, including one of the earliest and most effective COVID-19 vaccines.
Moderna's mRNA platform has enabled the development of therapeutics and vaccines for infectious diseases, immuno-oncology, rare diseases and autoimmune diseases. With a unique culture and a global team driven by the Moderna values and mindsets to responsibly change the future of human health, Moderna strives to deliver the greatest possible impact to people through mRNA medicines. For more information about Moderna, please visit modernatx.com and connect with us on X (formerly Twitter), Facebook, Instagram, YouTube and LinkedIn.
INDICATION
mRESVIA (Respiratory Syncytial Virus Vaccine) is a vaccine indicated for active immunization for the prevention of lower respiratory tract disease (LRTD) caused by respiratory syncytial virus (RSV) in adults 60 years of age and older.IMPORTANT SAFETY INFORMATION
Contraindications
Do not administer mRESVIA to individuals with a history of severe allergic reaction (e.g., anaphylaxis) to any component of mRESVIA.Warnings and Precautions
Management of Acute Allergic Reactions: Appropriate medical treatment must be immediately available to manage potential anaphylactic reactions following administration of mRESVIA.
Syncope: Syncope (fainting) may occur in association with administration of injectable vaccines, including mRESVIA. Procedures should be in place to avoid injury from fainting.
Altered Immunocompetence: Immunocompromised individuals, including those receiving immunosuppressive therapy, may have a diminished immune response to mRESVIA.
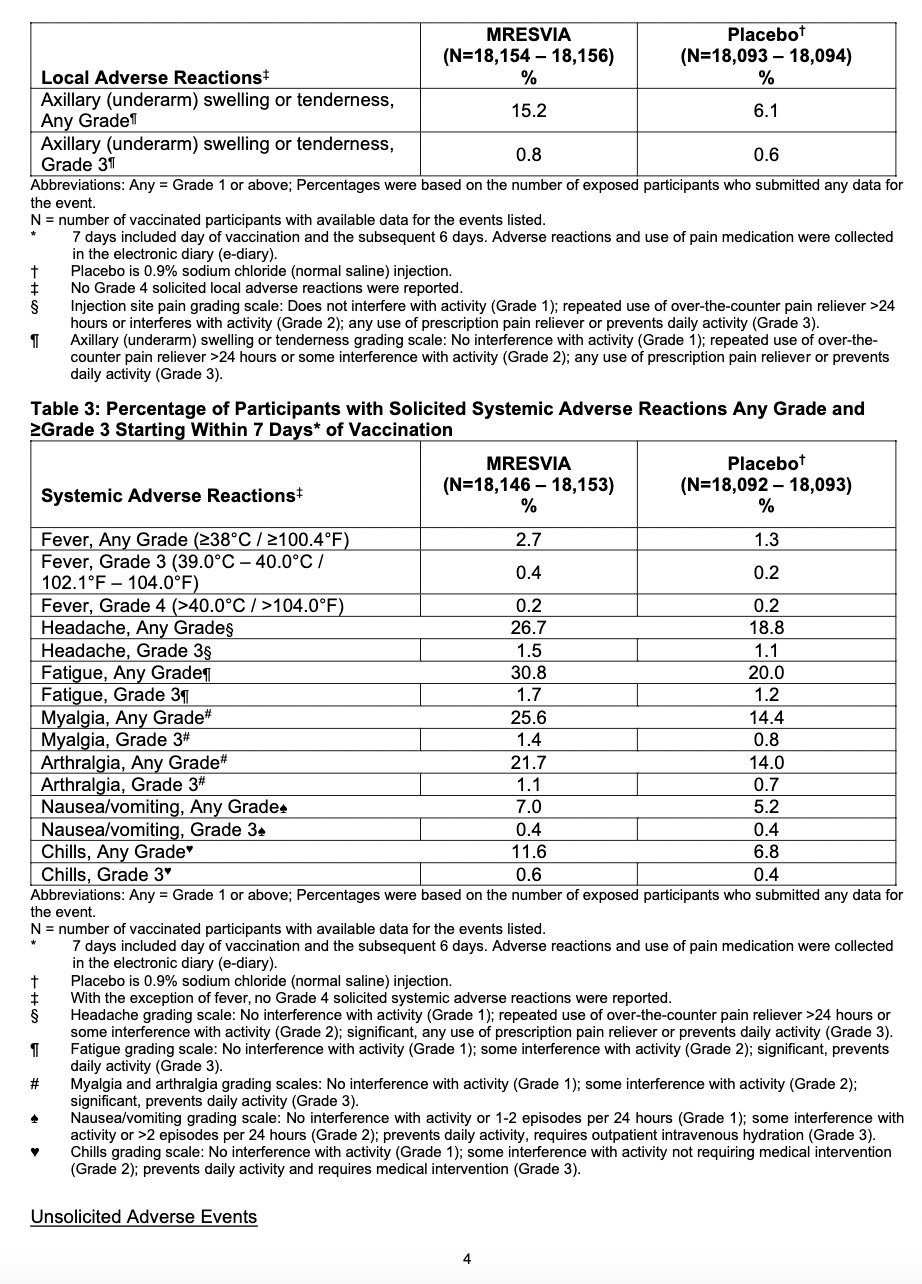
Adverse Reactions
In a clinical trial, the most commonly reported (≥10%) adverse reactions were injection-site pain (55.9%), fatigue (30.8%), headache (26.7%), myalgia (25.6%), arthralgia (21.7%), axillary (underarm) swelling or tenderness (15.2%) and chills (11.6%).To report suspected adverse reactions, contact ModernaTX, Inc. at 1-866-663-3762 or VAERS at 1-800-822-7967 or www.vaers.hhs.gov.
Please click for mRESVIA Full Prescribing Information.
Moderna Forward-Looking Statements
This press release contains forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995, as amended, including statements regarding: the vaccine efficacy and safety of mRNA-1345; the potential for mRESVIA to reduce disease burden from RSV; Moderna's pending marketing authorization applications for mRNA-1345; and Moderna's expectation to have mRESVIA available for eligible populations in the U.S. by the 2024/2025 respiratory virus season. The forward-looking statements in this press release are neither promises nor guarantees, and you should not place undue reliance on these forward-looking statements because they involve known and unknown risks, uncertainties, and other factors, many of which are beyond Moderna's control and which could cause actual results to differ materially from those expressed or implied by these forward-looking statements. These risks, uncertainties, and other factors include, among others, those risks and uncertainties described under the heading "Risk Factors" in Moderna's Annual Report on Form 10-K for the fiscal year ended December 31, 2023, and in subsequent filings made by Moderna with the U.S. Securities and Exchange Commission, which are available on the SEC's website at www.sec.gov. Except as required by law, Moderna disclaims any intention or responsibility for updating or revising any forward-looking statements contained in this press release in the event of new information, future developments or otherwise. These forward-looking statements are based on Moderna's current expectations and speak only as of the date of this press release.
Moderna Contacts
Media:
Elise Meyer
Senior Director, Corporate Communications
+1 617-852-7041
Elise.Meyer@modernatx.comInvestors:
Lavina Talukdar
Senior Vice President & Head of Investor Relations
+1 617-209-5834
Lavina.Talukdar@modernatx.com[1] https://www.cdc.gov/rsv/high-risk/older-adults.html
SOURCE: Moderna, Inc.
~~~
Website link: https://investors.modernatx.com/news/news-details/2024/Moderna-Receives-U.S.-FDA-Approval-for-RSV-Vaccine-mRESVIAR/default.aspx
Start talking to your kids in school, pastors, and co-workers. Keep an eye out for elders in nursing homes - start volunteering there or visiting more often! This jab is scheduled to come out in September-January, so there's bound to be more hype before then, and it will focus on our elders.
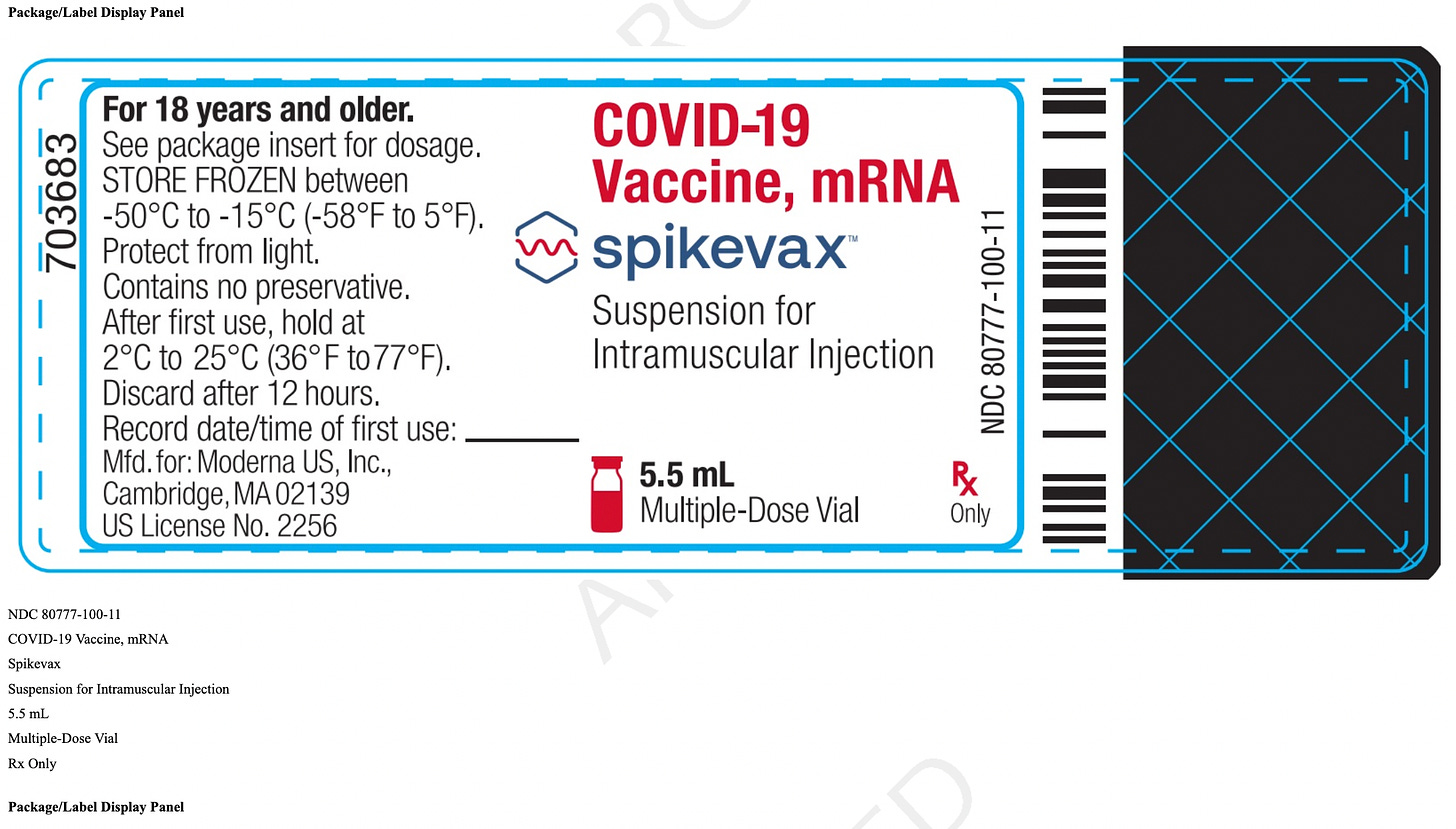
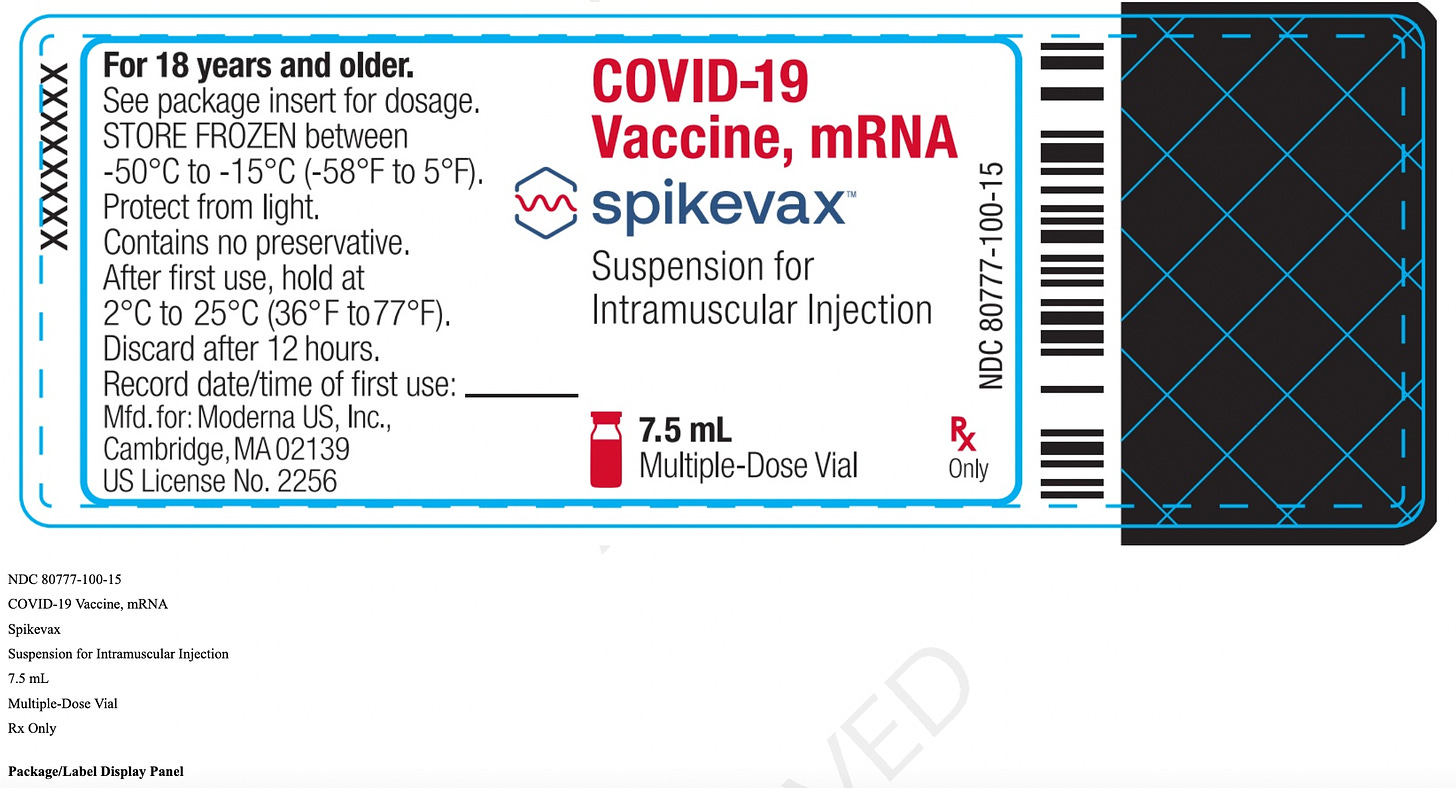
mResvia®: A Single-Use, Prefilled Syringe
Under the claim it is “saving vaccinators’ time and reducing the risk of administrative errors”, Moderna produced a single-dose jab that is “prefilled” in one syringe. No more pulling out your own syringe, filling it from a large multi-dose vial, and reusing the vial until it's empty! While it makes it much easier to perform the jab, it is sure to be much more costly!
What the CDC Had to Say About the Regular RSV Vax:
This is my recap of their website information on RSV. I paraphrase and incorporate WHAT I REALLY THINK below, and then I cite the CDC website for you as a Reference.
Bear in mind that the regular RSV vax had an increased incidence of Guillain-Barré Syndrome for those over age 60 - and they failed to notify the public or doctors in a timely manner.

God bless you and may you know that we are all in this together. No matter what they throw at us, NO ONE can HEAL and CURE better than God Himself! This is what we have learned, RIGHT?: » Trust Only in God, Not in Man! «
The first thing I note on the CDC RSV Vax site is that it was last updated on October 13, 2023. The vaccine information is on the “regular” vaccine, not the mRNA version. Nevertheless, we glean important information on RSV and vaccines.
RSV IS A MILD COLD that presents with “mild, cold-like symptoms”;
Infants and elders in nursing homes are at increased risk, especially if chronically sick;
You can catch RSV by touching a doorknob; Good - let me get it right away so I can have antibodies. Or better yet, let me touch doorknobs and NOT get sick because God protects me!
It usually lasts 1-2 weeks; but watch for low oxygen levels that can lead to 👉👉👉 PNEUMONIA, HOSPITALIZATION, OR DEATH 👈👈👈 …
✋(CODE = Hospital Killing Protocols)⛔️ »Watch Out!« !!! Remember to ALWAYS Look at the Patient (and not the pulse oximeter) before considering an ER visit!
Many have made this mistake and ended up with DEAD PARENTS and KIDS after trusting the murderous WHITE COATS that put them on Remdesivir, a ventilator, proclaimed them FIRST a “DNR” and THEN murdered them with DEATH ROW DRUGS! BEWARE! See my Guidebook to Covid Care that includes the Hospital Killing Protocols and a sample Medical Directive to cover yourself (but BEAR IN MIND that hospitals have put patients on Remdesivir, ventilators, and the protocol WITHOUT their permission - and these patients have DIED!).
» Check the cases of hospital murders. Ask Scott Schara of, , and Debrah Bucko’s family.
» STAY OUT OF THE HOSPITAL!
» Get the Death by Hospital Killing Protocol App on your cell! For iPhones: https://apps.apple.com/us/app/dbhp/id6478464423 and for Android Phones: https://play.google.com/store/apps/details?id=com.letsol.apps.dbhp
» Be sure to have my Medical Directives - or those from a reference of your choice - and someone in the room with you, if you need hospitalization for any reason.
INFANTS GET RSV ANTIBODIES THROUGH THEIR MOTHERS who got the regular RSV jab while pregnant; {look at 3 icons that show up with the word pregnant 🤰 pregnant 🫄 pregnant 🫃‼️ }; If infants get antibodies from their mothers, then why don’t mothers (and everyone else) get natural immunity for themselves, after getting sick?
The CDC wants “pregnant people” to get the regular RSV shot at 32-36 weeks of pregnancy {I will say here that only women can get pregnant, so this applies to pregnant 🤰 women};
You can get the regular RSV jab at the same time you get other vaccines.💉 vaccine 💉 💉 💉 {Why? So we don't know which one gave you Guillain-Barré Syndrome?}
They insinuate you may have trouble with the regular RSV jab if you were allergic before, or have any “life-threatening allergy” (CODE = Anaphylactic Reaction; most require an epinephrine shot as with a bee 🐝 sting); but they insisted you could just get the Covid jab, EVEN IF you had bee sting allergies, and many doctors told their patients to “just bring your EpiPen”; THAT was MALPRACTICE and it DID HARM. Once again, common sense prevails - don’t let them kill you with ANY jab if you have anaphylaxis against ANYTHING!
The CDC says “your health care provider may decide to postpone RSV vaccination until a future visit.” So if you go to the doctor and have a conversation about your anaphylaxis for anything, your doctor can decide that today isn’t a good day for you to get ANY jab. That’s what the CDC says, and you can tell them it’s RIGHT HERE on their website!
To cut down your chance of allergic reaction, you can take Benadryl® or generic diphenhydramine, antihistamines (Claritin;®, Allegra®, and more many people take 2-3 different ones a day for Mast Cell Activation Syndrome), and ice your arm before and afterwards to slow down the blood flow to the arm. And of course, have your EpiPen® ready.
You can get the regular RSV jab even if you are mildly sick with a cold, or you can just wait until you are well - but if you are moderately or severely sick don’t get it then!
» I will insert here that maybe there's going to be a thing called, “LONG RSV” from the mRNA RSV vax. Here, people would stay sick for a long time after the jab, which will STILL make them sick, and then the real problem will be a post-mRNA RSV vax injury «
Regular RSV Vax: Risks and Adverse Events
The CDC has these statements on the regular RSV vax, which helps us know more about how they think. Remember that any Informed Consent for any drug or procedure should include BOTH 1) the general side effects for everyone, AND 2) those that may be more likely to happen in you, considering your diagnoses.
You can generally suffer from the regular RSV vax:
Diarrhea
Fatigue
Fever
Headache
Joint pain
Muscle pain
Nausea
Pain on arm
Redness on arm
Swelling on arm
Serious Adverse Events
1. Guillain-Barré Syndrome (GBS) was more frequently reported in older adults after the RSV jab was given in the clinical trial. As stated earlier, this was hidden for some time.
Preterm birth during pregnancy.
High blood pressure during pregnancy, including pre-eclampsia. They don't know if the vax caused it;
Lightheadedness or fainting. Don't stand up fast after a jab, IF you're ever going to get one;
Vision changes 👀 . That could be white spots that disappear, blurry vision, or blindness… they don't say what it is; 🤷♀️
Ringing in the ears. This has been a big problem for many, since the Covid jab.
And I quote:
“As with any medicine, there is a very remote chance of a vaccine causing a severe allergic reaction, other serious injury, or death.”
What if there is a serious problem?
An allergic reaction could occur after the vaccinated person leaves the clinic.
If you have had anaphylaxis to any jab, or have anaphylaxis from anything, you should never drive by yourself to get a jab. IF you feel forced into it, first try to run away. Otherwise, most clinics will have you stay in clinic for at least an hour, for observation. Have antihistamines and an EpiPen® your person.
If you see signs of a severe allergic reaction (hives, swelling of the face and throat, difficulty breathing, a fast heartbeat, dizziness, or weakness), call 9-1-1and get the person to the nearest hospital.
For other signs that concern you, call your health care provider.
Adverse reactions should be reported to the Vaccine Adverse Event Reporting System (VAERS). Your health care provider will usually file this report, or you can do it yourself. Visit the VAERS website or call 1-800-822-7967. VAERS is only for reporting reactions, and VAERS staff members do not give medical advice.
How can I learn more?
Ask your health care provider.
Call your local or state health department.
Visit the website of the Food and Drug Administration (FDA) for vaccine package inserts and additional information.
Contact the Centers for Disease Control and Prevention (CDC):
Call 1-800-232-4636 (1-800-CDC-INFO) or
Visit CDC’s vaccine website
Many vaccine information statements are available in Spanish and other languages. See www.immunize.org/vis
Hojas de información sobre vacunas están disponibles en español y en muchos otros idiomas. Visite www.immunize.org/vis
Vaccine Information Statement
RSV Vaccine
(10/19/2023)Department of Health and Human Services
Centers for Disease Control and PreventionSource: https://www.cdc.gov/vaccines/hcp/vis/vis-statements/rsv.html
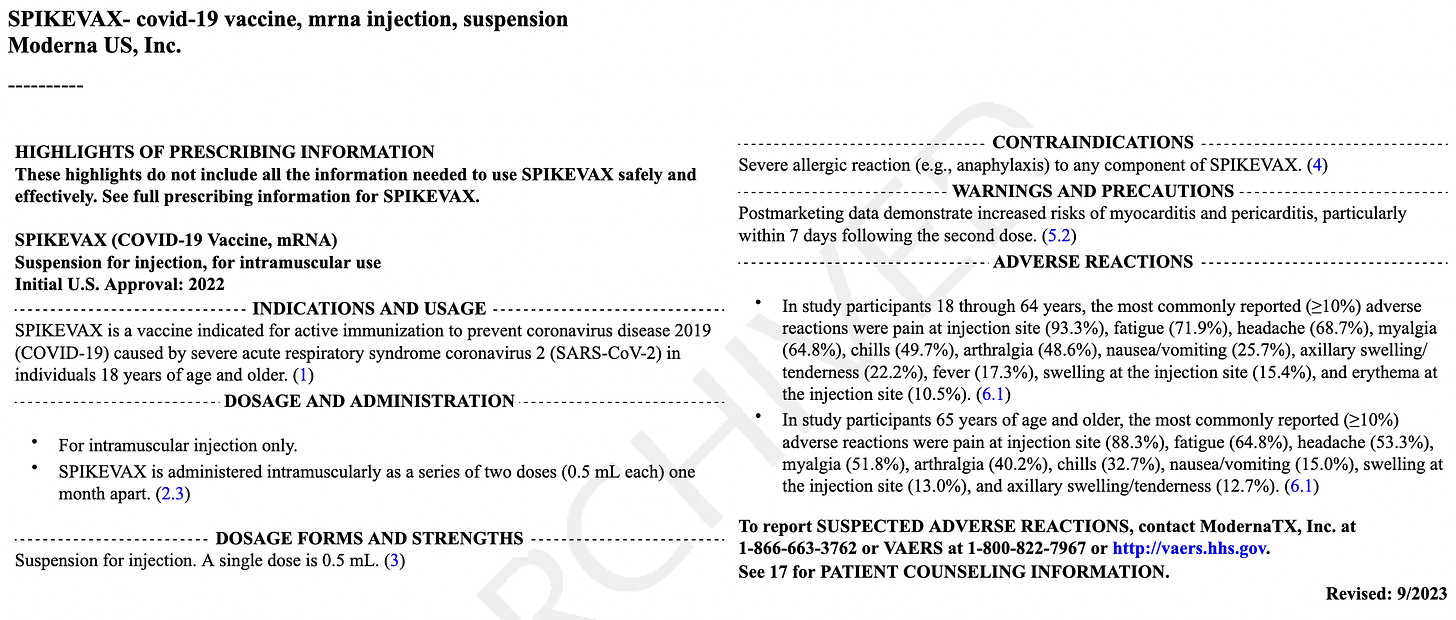
The Package Insert for mRESVIA®, the Moderna mRNA RSV Vax
Below is the package insert for your review, noting that there were just BLANK PAGES stating, “Page left intentionally blank” provided for the mRNA Covid jab. Senator Ron Johnson was presented with the very package insert, as seen by my screenshot from my December 7, 2022 article:
Key Package Insert Points:
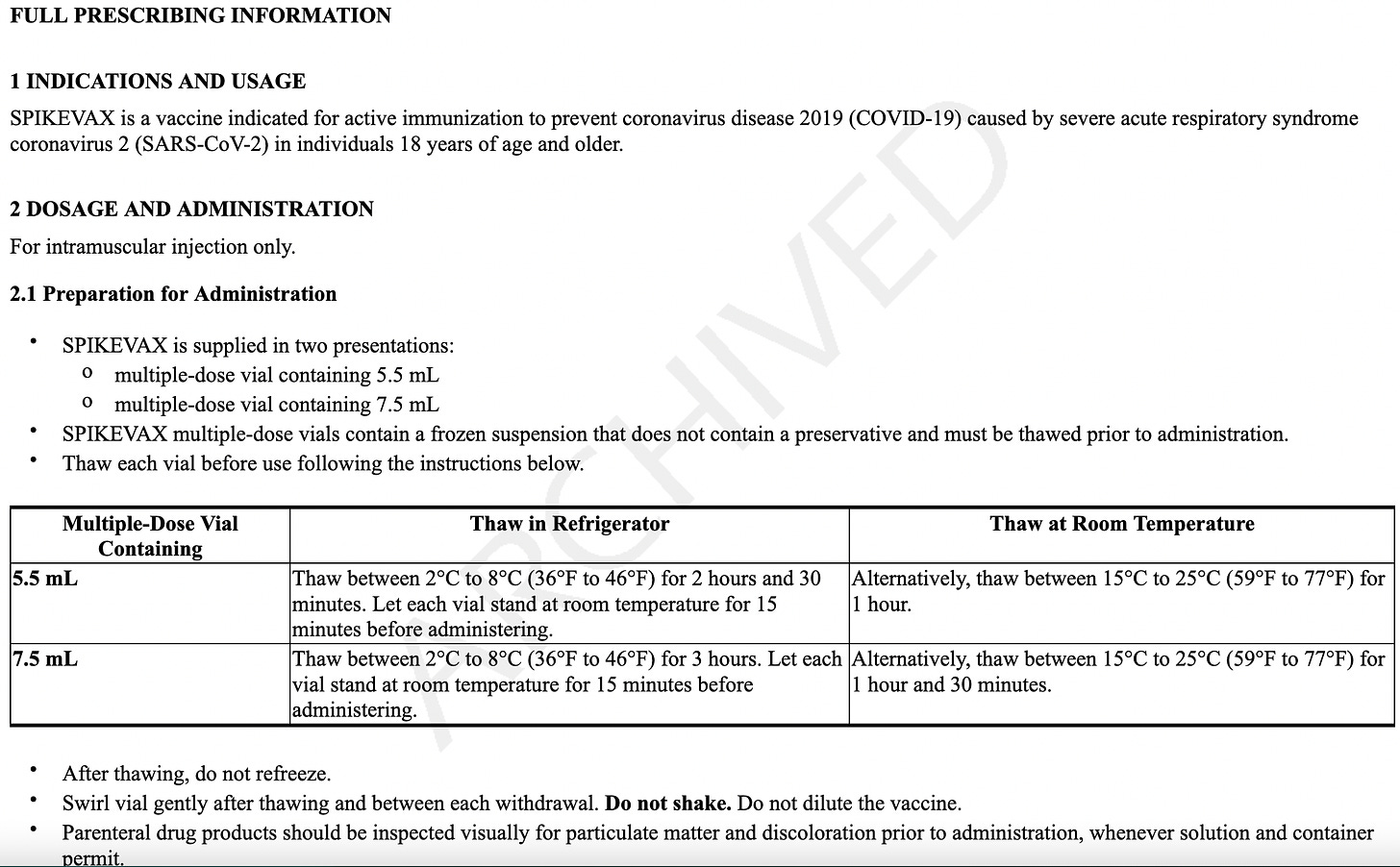
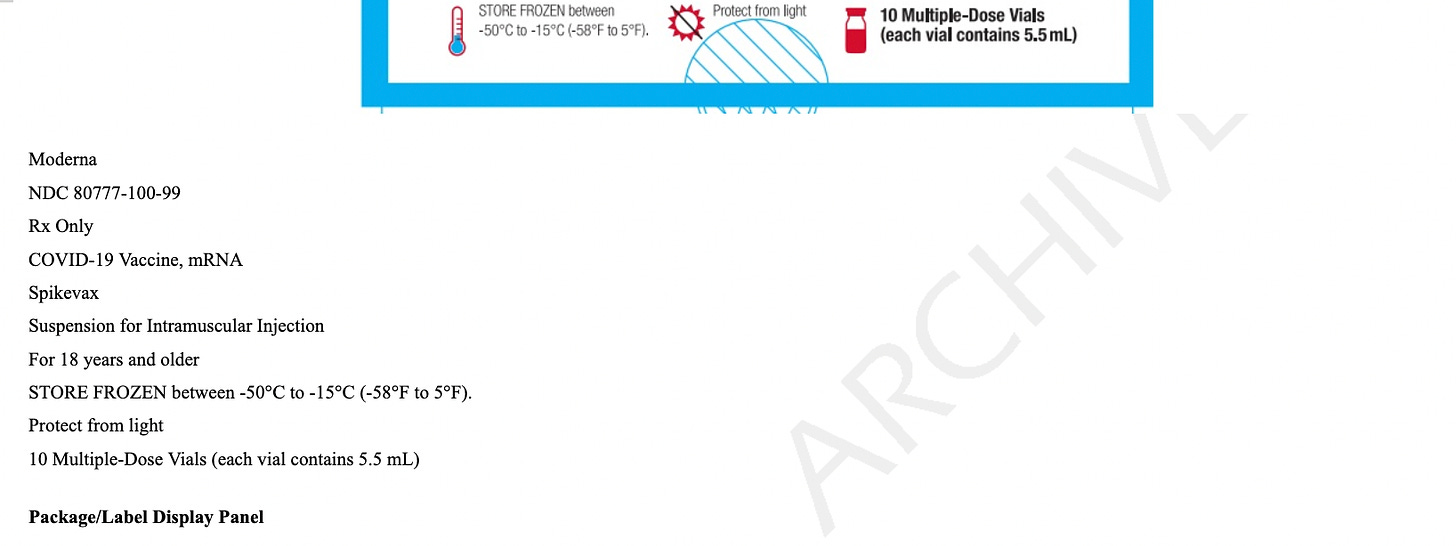
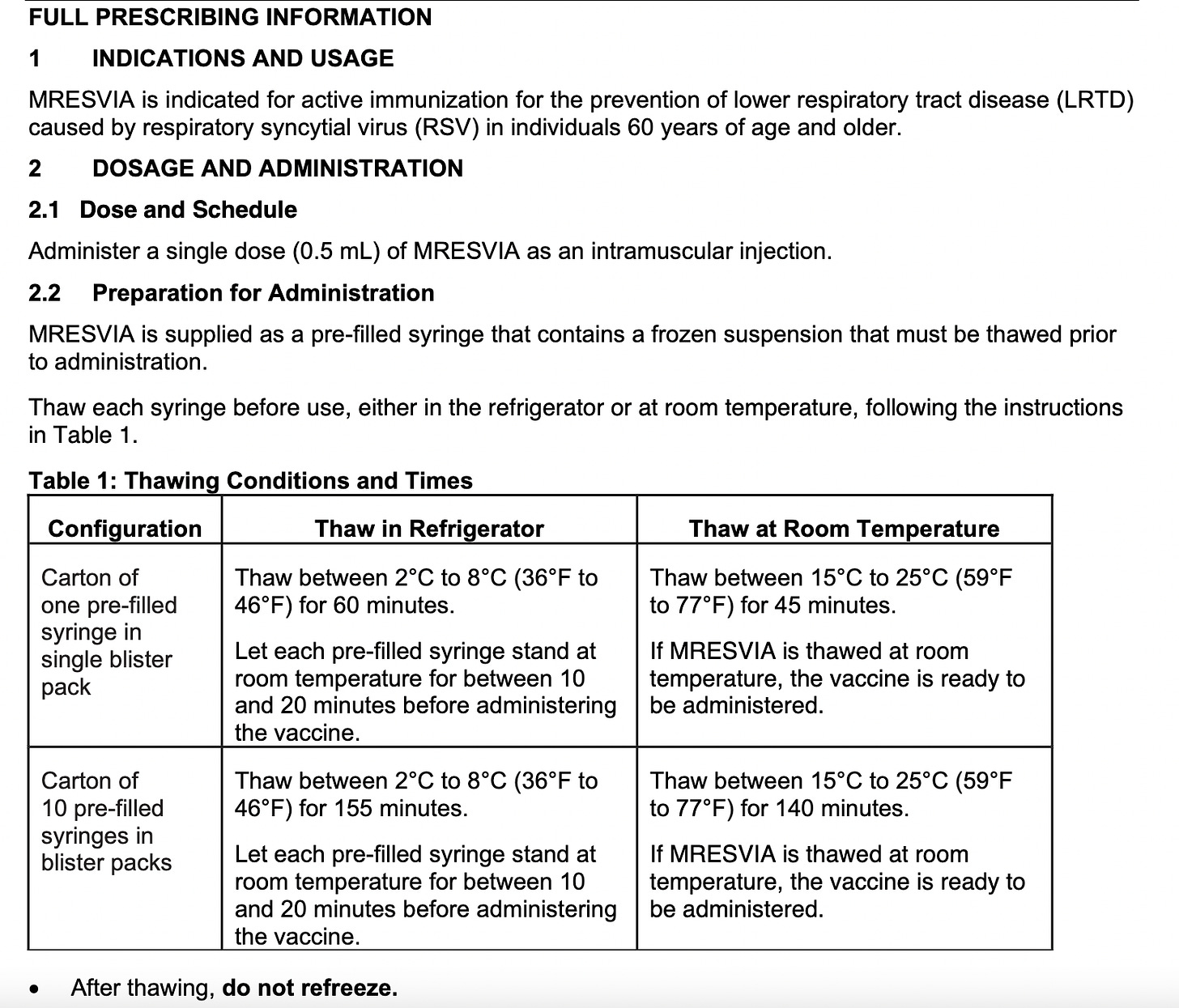
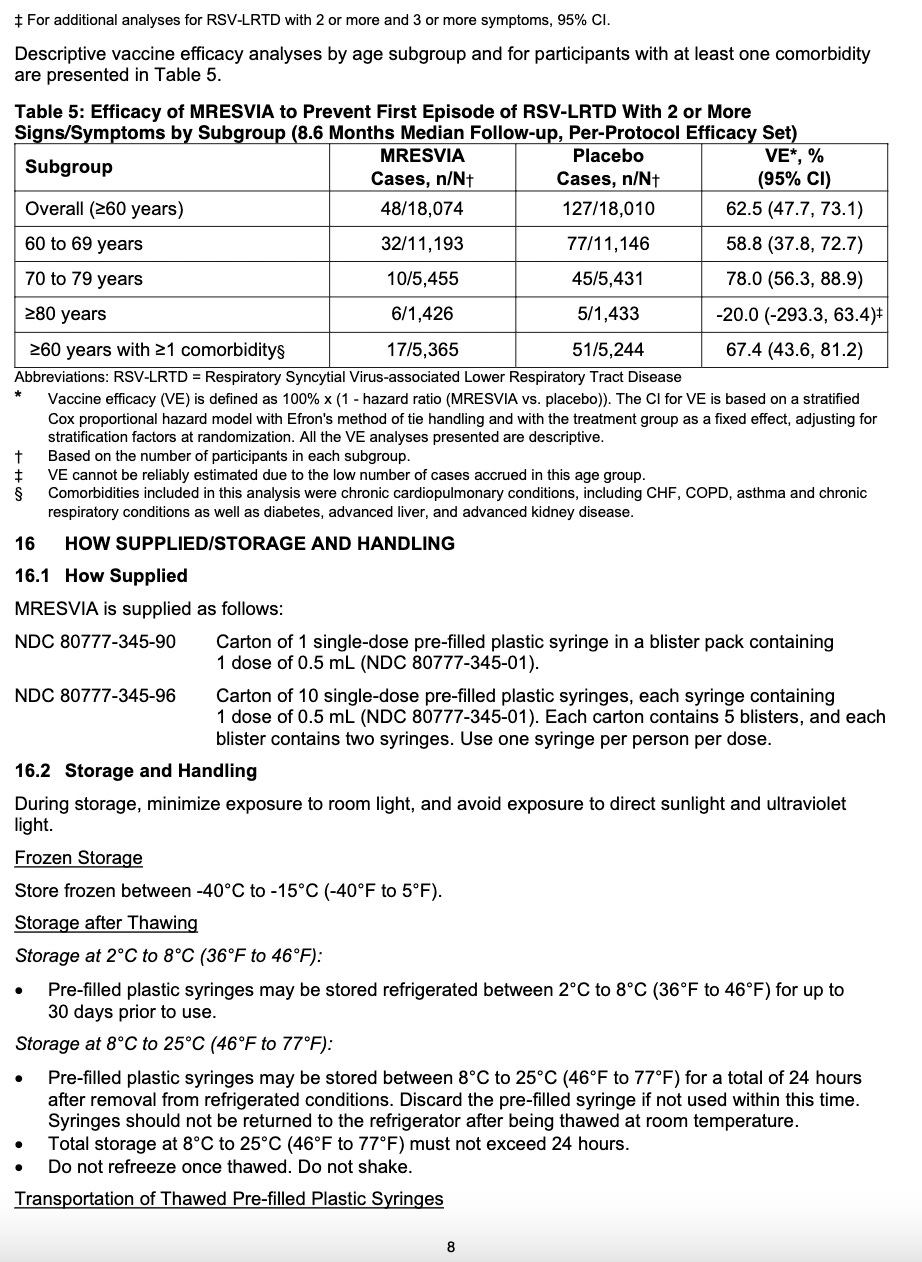
It is frozen and must be thawed and not re-frozen. If you see someone shaking them, they’re NOT supposed to do that. They can be thawed in the refrigerator or a room temperature, but if done at room temperature, they must be DISCARDED and not refrigerated. If refrigerated or kept at 46°F to 77°F, they last 24 hours. It is a white or off-white solution that can have clear particles in it - if it’s any other color, it’s trash.
Do not administer MRESVIA® to individuals with a history of a severe allergic reaction (e.g., anaphylaxis) to any component of MRESVIA® [see Description (11).
FDA Approved for those 60 and older, to prevent RSV from going to lower lung infection - even though THERE IS NO TEST FOR RSV. Like Covid, RSV is a COLD. THE PCR IS NOT FOR DIAGNOSIS and says you are pregnant when you have a part of a pregnancy marker in your system. That’s a HUGE mistake to make 97% of the time, the failure rate of the test.
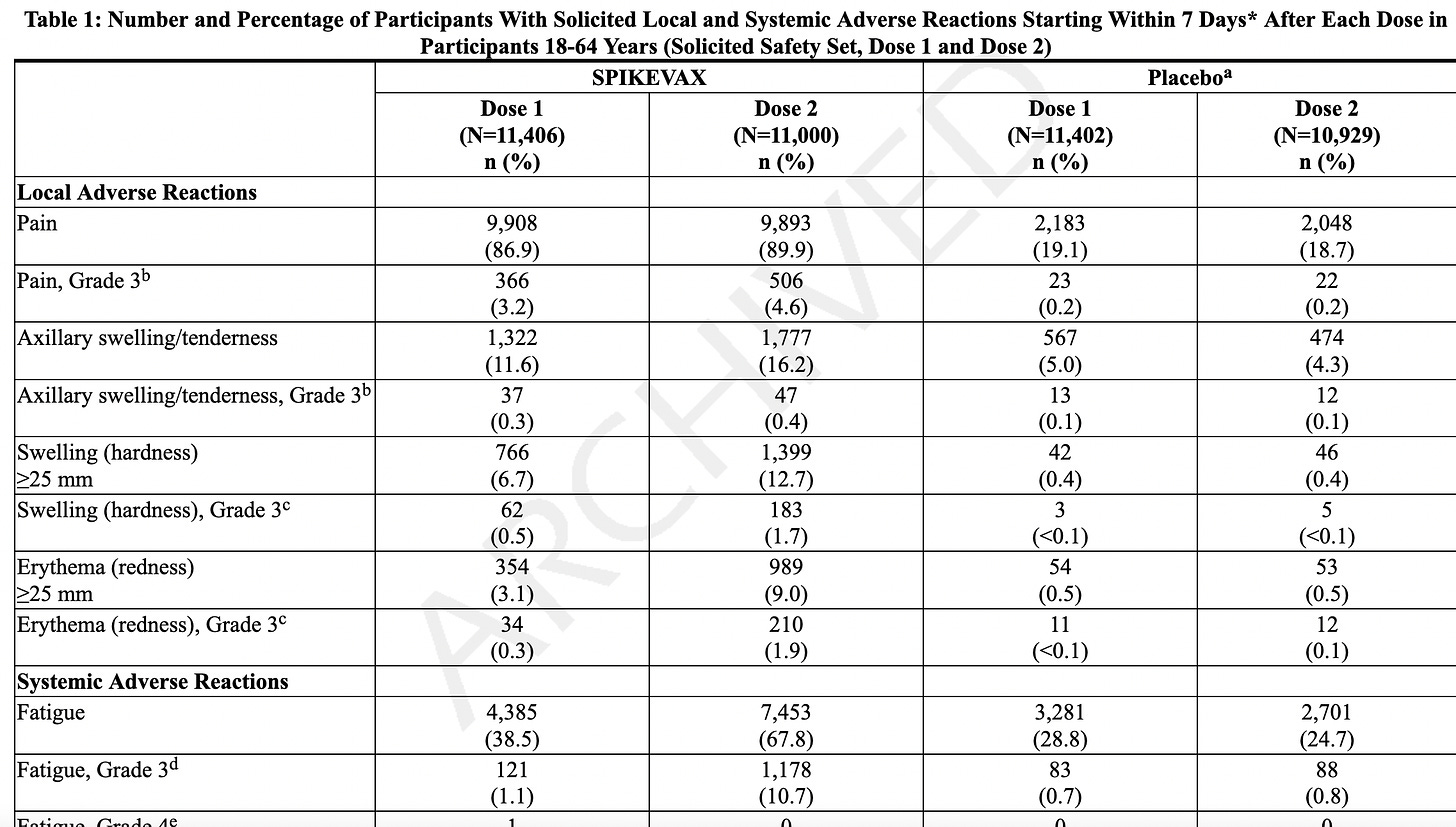
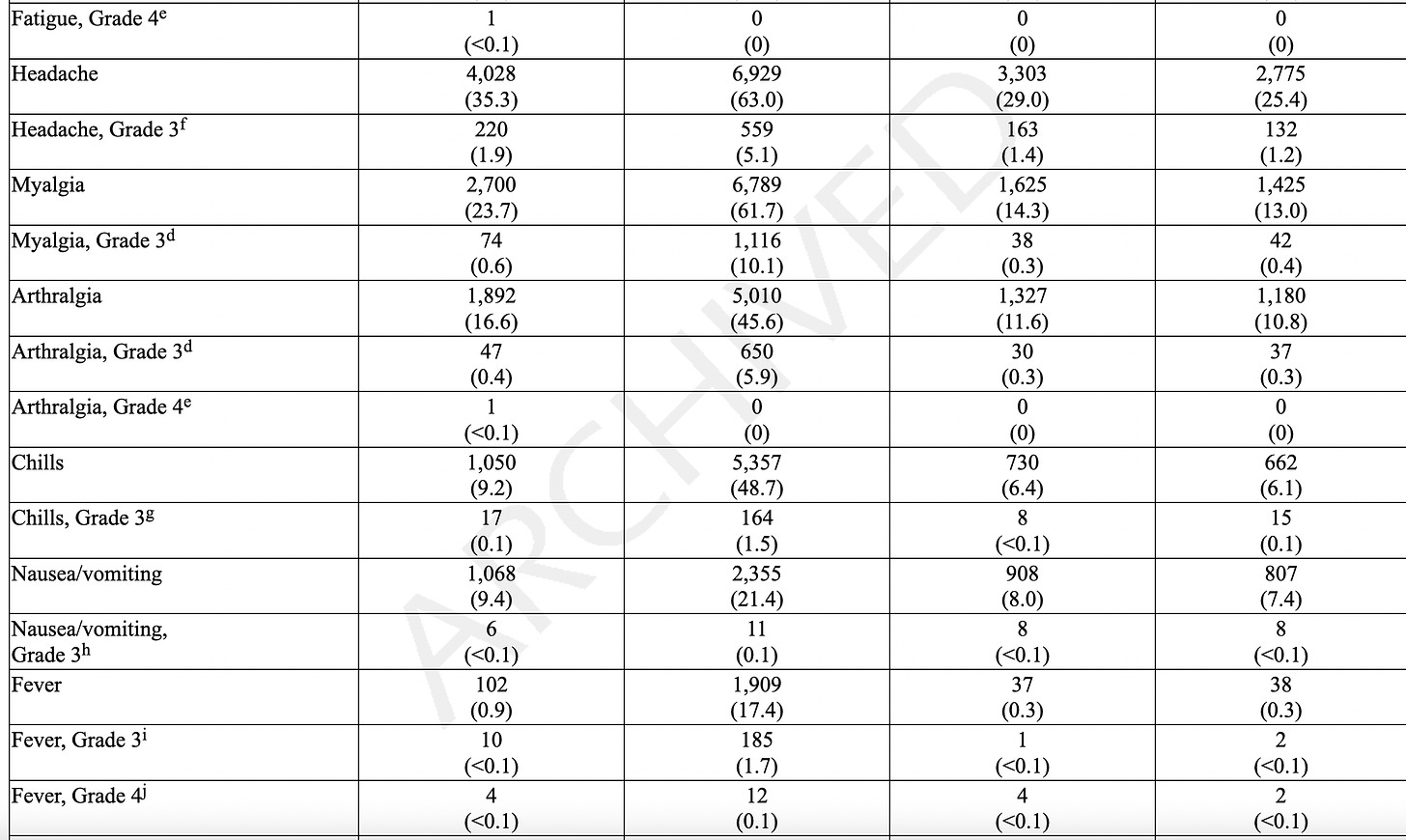
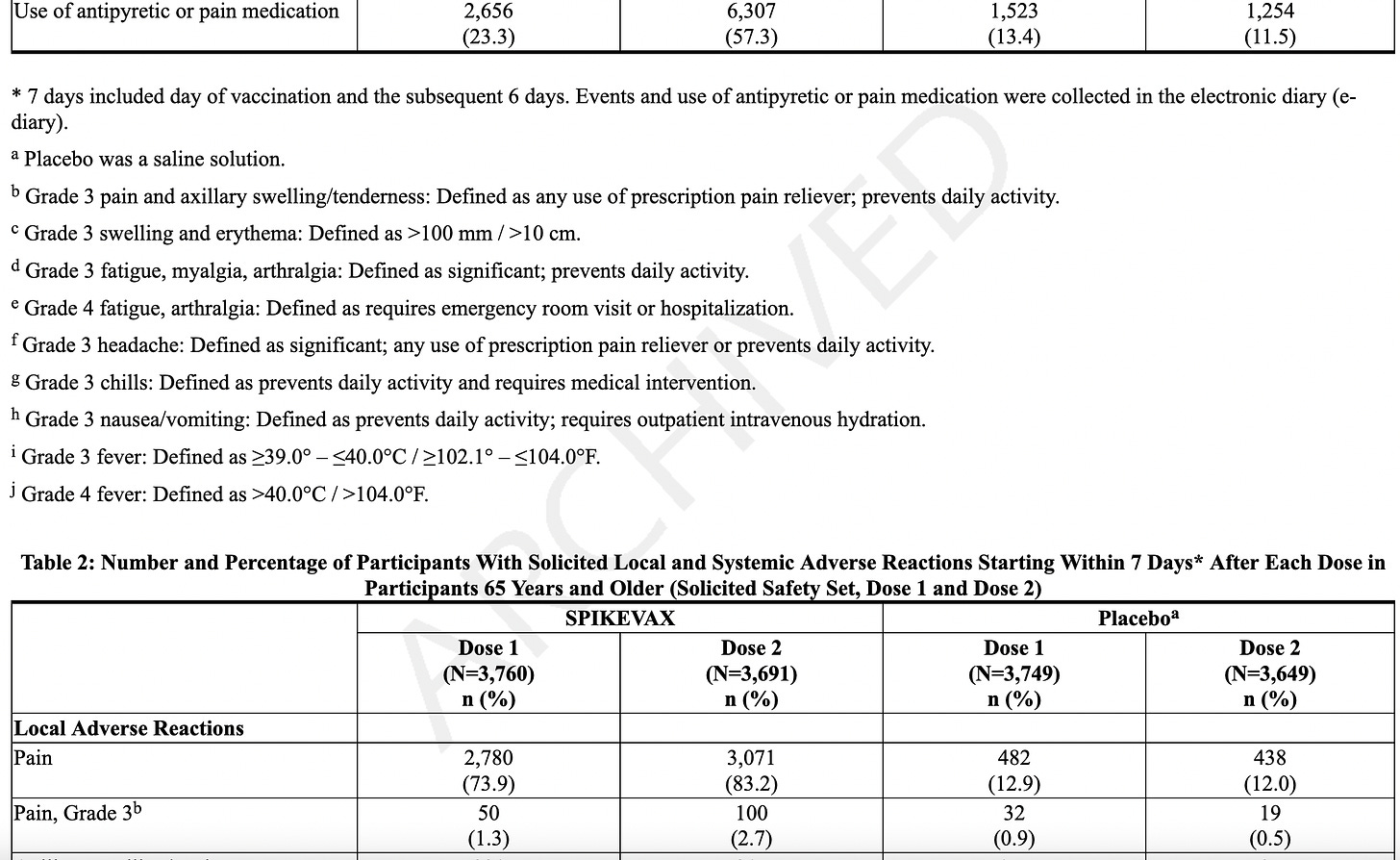
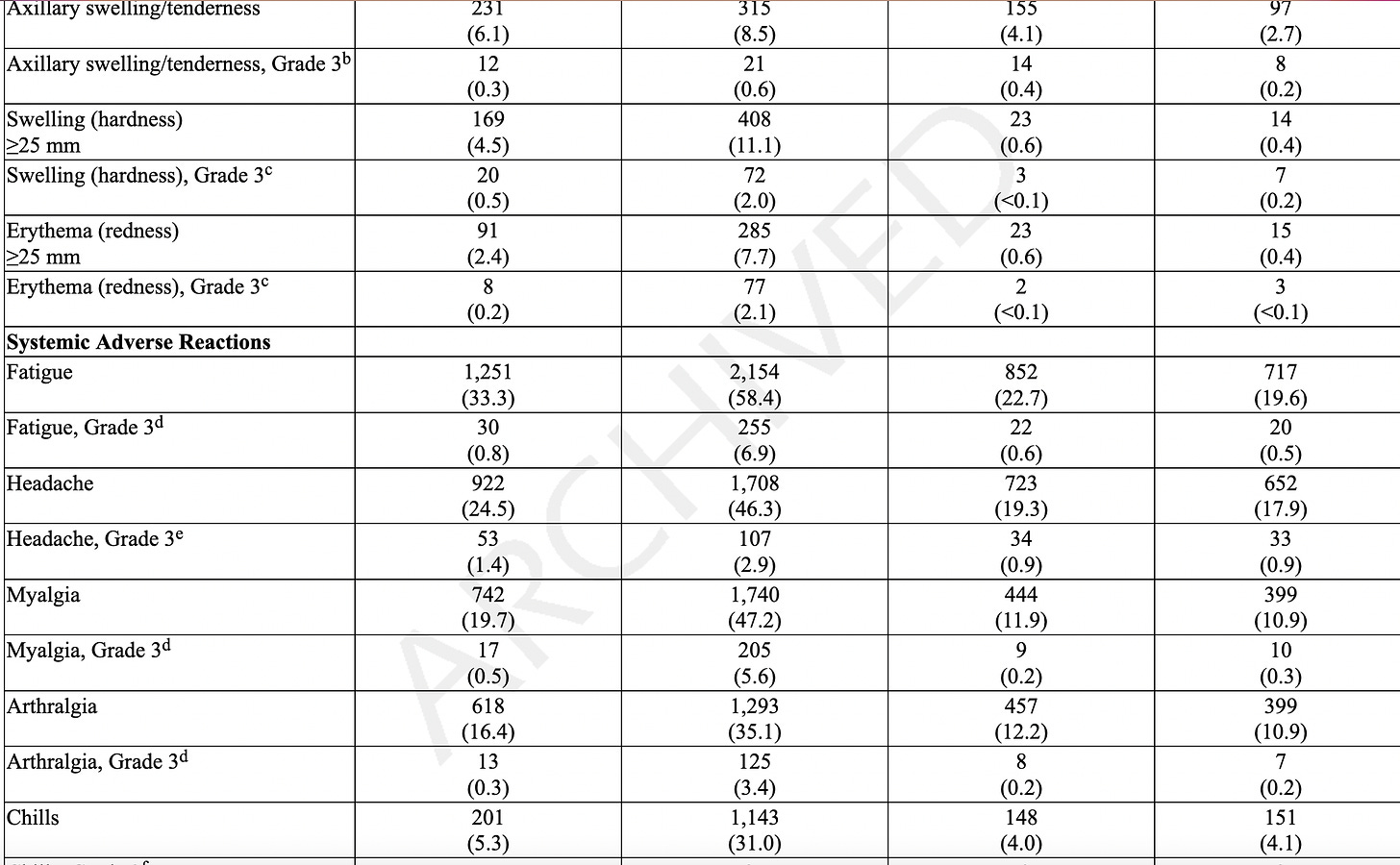
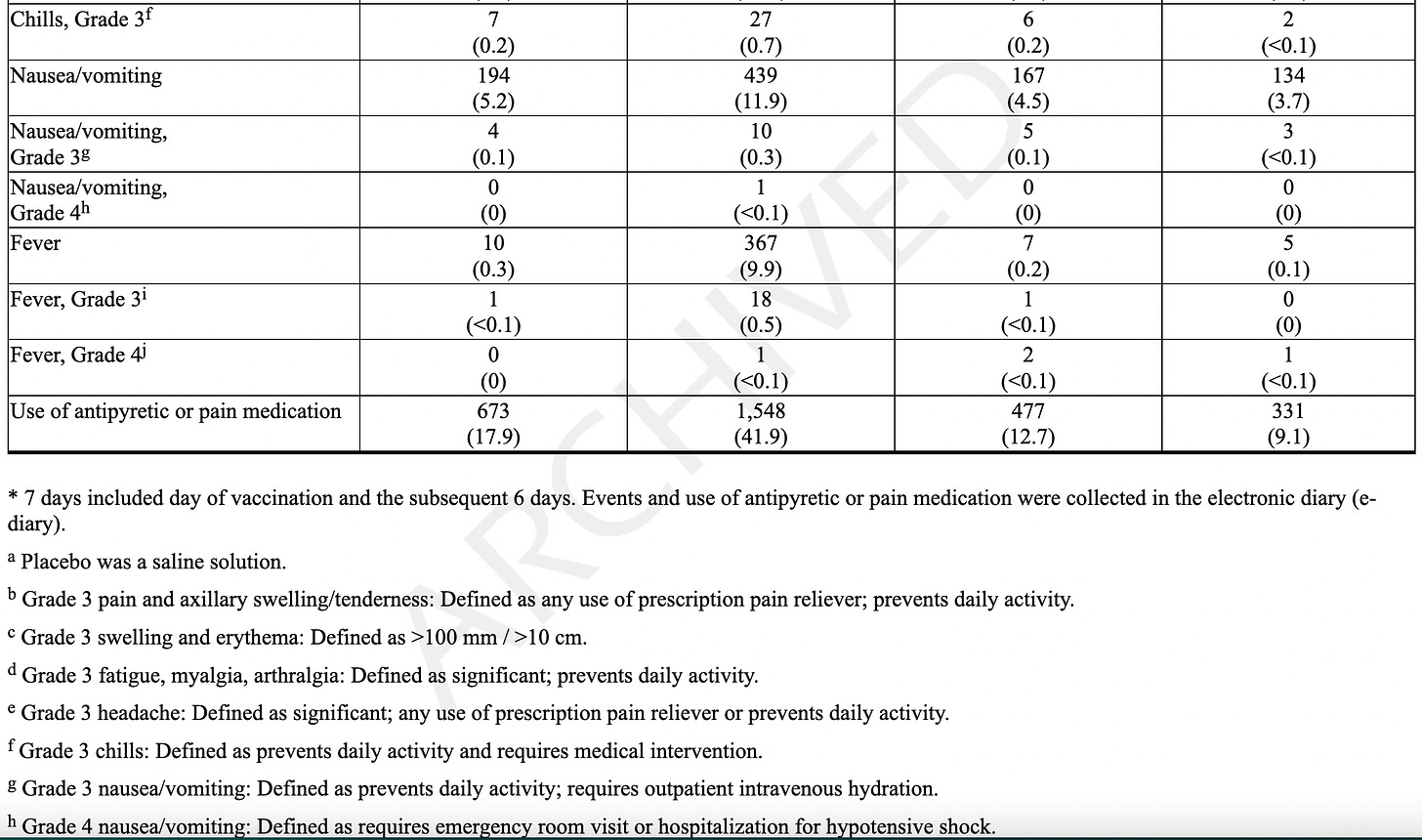
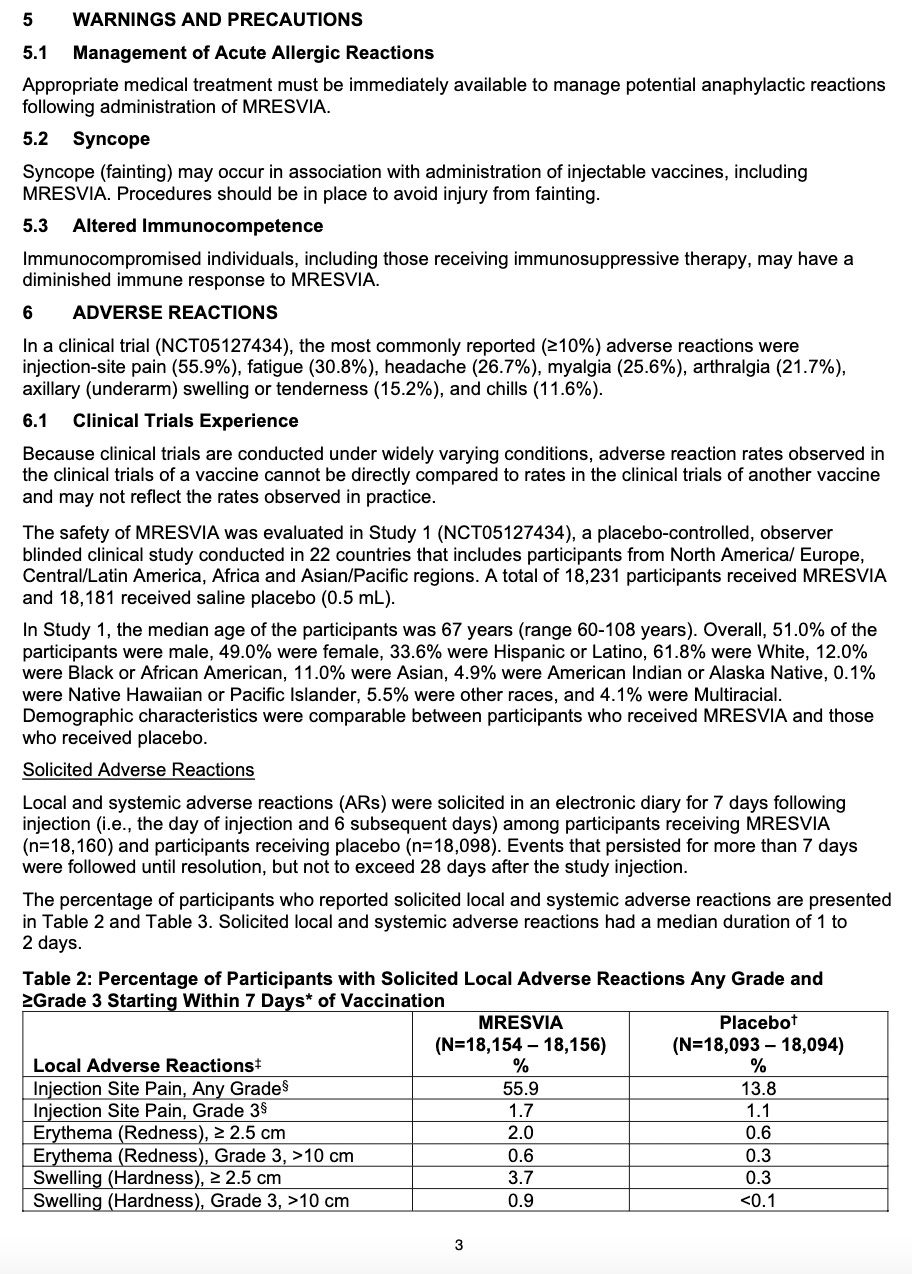
Adverse reactions. In a clinical trial (NCT05127434), the most commonly reported (≥10%) adverse reactions were injection-site pain (55.9%), fatigue (30.8%), headache (26.7%), myalgia (muscle soreness) (25.6%), arthralgia (joint soreness) (21.7%), axillary (underarm) swelling or tenderness (15.2%) AS IT TRAVELS THROUGH YOUR LYMPH NODES and your body tries to get rid of it, and chills (11.6%). Other “unsolicited” adverse events included nausea/vomiting and urtricaria (allergic hives).
Like the Covid jab, the shot travels and does not stay in the arm. Adverse events can cause you to miss work or school, and necessitate a return visit to your doctor. We can assume it goes to the blood stream, heart, ovaries, testes, kidneys, liver, brain, and all organs.
The “safety” of synthetic MRESVIA® was evaluated in Study 1 (NCT05127434), a placebo-controlled, observer-blinded clinical study conducted in 22 countries that includes participants from North America/ Europe, Central/Latin America, Africa and Asian/Pacific regions. A total of 18,231 participants received MRESVIA and 18,181 received saline placebo (0.5 mL).
They only followed patients for 7 days using an electronic diary. If they were sick beyond that, they stopped following adverse events at 28 days, even though as stated later, they “followed” people for an average of 311 days!
The AVERAGE adverse event lasted 1-2 days. We don’t know the range, which would include the shortest and the longest duration, but we know that the study stopped tracking adverse events 28 days.
How many people are still PERMANENTLY sick from the jab? We don’t know. And how will they be in 1 month or 1 year? No one knows. And bear in mind that routine vax approval requires two to three years of observation - PLUS genetic transfer technology observation for 5 to 15 years.
Serious Adverse Events (SAE). They state that the median duration of “safety follow-up” was 311 days (range 1 to 585 days), with 96.6% having a 6-month follow-up and about a 7.85% SAE rate in both groups. SAEs “were reported by” (not “occurred in” - note that some participants may not have WANTED to complain about adverse events, and the study did NOT call everyone or require them to answer daily questionnaires, a study fault. Some SAEs:
a. Facial paralysis. By one patient 4 days after jab, from MRESVIA®. This gets a bit murky because it then states,
“Within 28 days and 42 days post vaccination, there was no imbalance in reports of facial paralysis (including Bell’s palsy) between treatment groups. There were no other notable patterns or numerical imbalances between treatment groups for specific categories of serious adverse events that would suggest a causal relationship to MRESVIA.”
But how can they compare “both groups”, if only ONE patient complained? Or how many more DID complain? We don’t know.
Pregnancy. Thankfully, they didn’t jab any pregnant women. They did rat studies:
“A developmental toxicity study was performed in female rats administered a vaccine formulation that included approximately twice the amount of nucleoside-modified messenger ribonucleic acid (mRNA), encoding the same RSV fusion (F) glycoprotein stabilized in the prefusion conformation, as in MRESVIA. The vaccine formulation was administered twice prior to mating and twice during gestation. The study revealed no evidence of harm to the fetus due to the vaccine (see Data).”
On RSV fusion (F) glycoprotein: Note that it causes the virion membrane to fuse with a target cell (human) membrane. This is reminiscent of the spike protein jab which also attaches to the human cell membrane and ramps up “spike protein” synthesis for an indefinite amount of time.
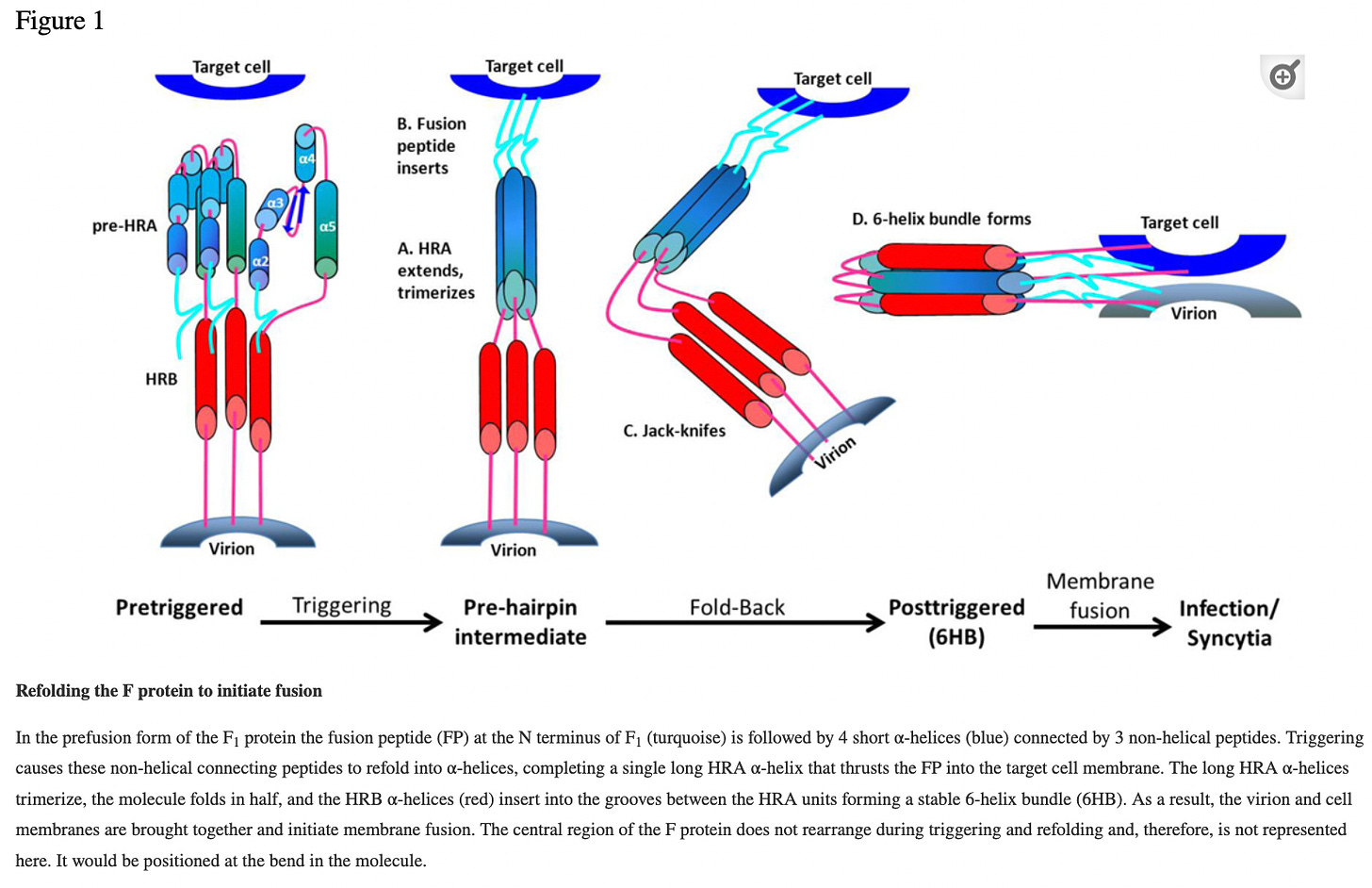
Abstract. The two major glycoproteins on the surface of the RSV virion, the attachment glycoprotein (G) and the fusion (F) glycoprotein, control the initial phases of infection. G targets the ciliated cells of the airways, and F causes the virion membrane to fuse with a target cell membrane. The F protein is the major target for antiviral drug development, and both G and F glycoproteins are the antigens targeted by neutralizing antibodies induced by infection. In this chapter we review the structure and function of the RSV surface glycoproteins, including recent X-ray crystallographic data of the F glycoprotein in its pre- and postfusion conformations, and discuss how this information informs antigen selection and vaccine development.
Source: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4211642/
Rat fetal malformation and infertility study. They studied rats 28 and 14 days prior to mating, and on gestation days 1 and 13, then stopped. They concluded there were no fetal abnormalities and no infertility. But we don’t know how many rats there were, how many didn’t get pregnant, and why they stopped at 13 days’ gestation. I can’t help but wonder if they saw some tumors in these rats, but ignored it because the tumors weren’t part of the study.
According to Terminix, “a female rat will have six to 10 babies at one time. They are born blind and without fur. The gestation period for rats is quite short – around three weeks for most species.” Here is my question: Just Why did they stop at 13 days, when a pregnancy lasts 21 days? Was it because ALL fetal abnormalities could be seen within 13 days, or because they were afraid the rats could not carry the fetus to term? Why didn’t they stop the study after birth?
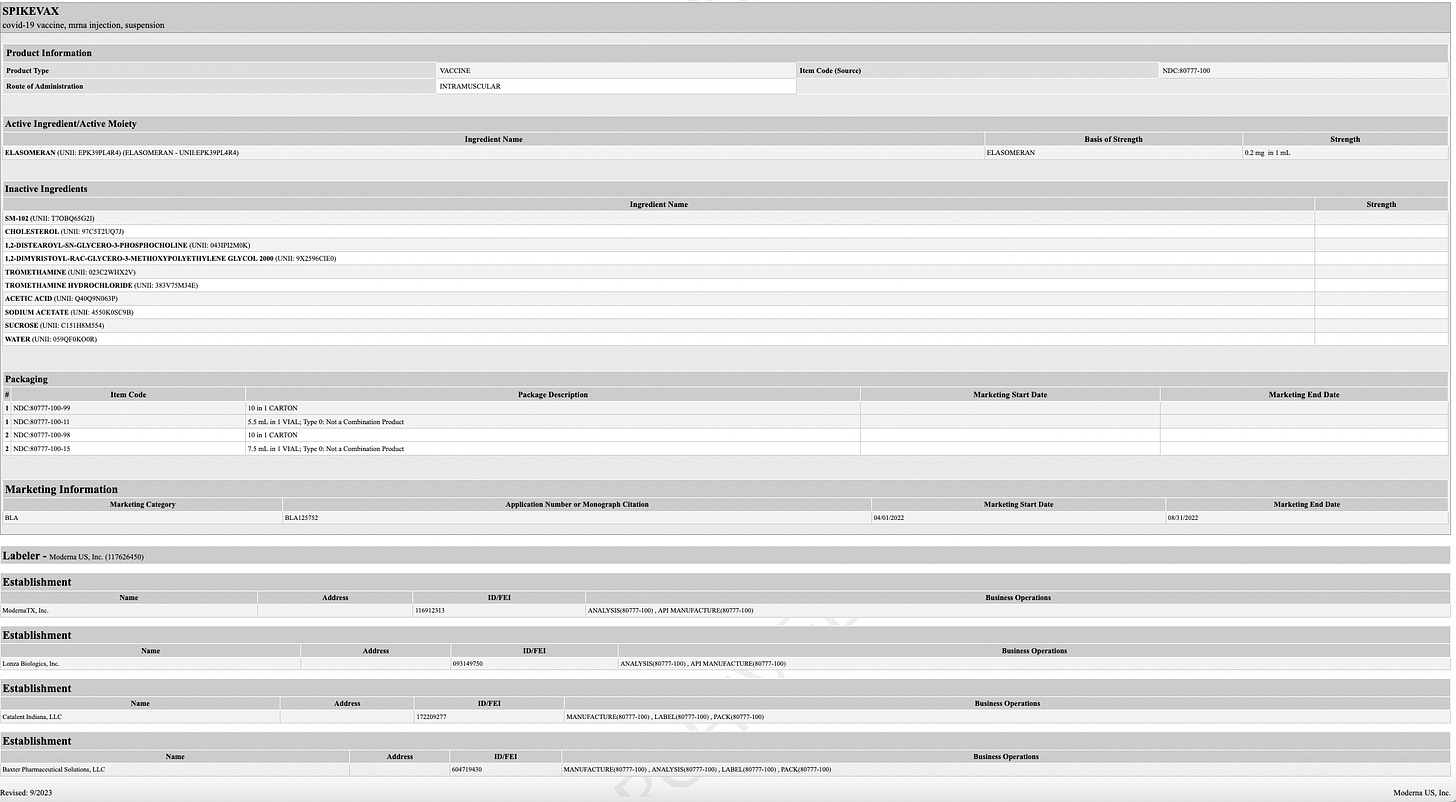
Contents. Each 0.5 mL dose of MRESVIA contains:
a. 50 mcg of nucleoside modified mRNA encoding the RSV F
glycoprotein stabilized in the prefusion conformation (pre-F protein);
b. A total lipid content of 1.02 mg (SM-102 (heptadecan-9-yl 8-((2-hydroxyethyl) (6-oxo-6-(undecyloxy) hexyl) amino) octanoate);
c. Polyethylene glycol (PEG): 2000 dimyristoyl glycerol [PEG2000-DMG]; d. Cholesterol;
e. 1,2-distearoyl-sn-glycero-3-phosphocholine [DSPC]);
f. 0.25 mg tromethamine;
g. 1.2 mg tromethamine hydrochloride;
h. 0.021 mg acetic acid;
i. 0.10 mg sodium acetate trihydrate;
j. 44 mg sucrose;
k. And water for injection.
l. There are no preservatives.
NOTE: There are no reports of DNA contamination, because the jab is not yet available.
Pediatric Use, Section 8.4, Moderna cites,
“Safety and effectiveness of MRESVIA in individuals younger than 18 years of age have not been established.”
This implies they used it on some 18-year olds and those under age 60. Awful.
Mechanism of Action, Section 12.1. They give one sentence here,
“MRESVIA induces an immune response against RSV pre-F protein that protects against LRTD caused by RSV.”
This is nowhere near a description of the mechanism of action from a normal drug. There is absolutely no detail here, no images or diagrams of binding sites on the cell surface, what compound gets upregulated like the “spike protein” was for the mRNA jab, nothing. How does one go from “an immune response” induction “against RSV pre-F protein”, to “protects against LRTD caused by RSV”? What’s in the middle?
Cancer and infertility. Moderna admits they haven’t even tested the jab’s effect on animals for cancer-causing ability or infertility:
“MRESVIA has not been evaluated for carcinogenic or mutagenic potential, or impairment of male fertility in animals.”
Clinical Study 1 (Section 14). They cite that in adults over age 60, with 35,064 participants who got either MRESVIA® (n = 17,561) or placebo (n = 17,503). They didn’t allow people to participate if they had myocarditis, pericarditis, or myopericarditis, autoimmune dysfunction, or any other serious vax reaction, or any other shot in the last 28 days…
I wonder why? If you had to sign a consent form to be in this trial, wouldn’t you think that the jab could CAUSE any of these?
Why aren’t cancer and genomic integration mentioned with the other known mRNA jab adverse events?
There’s more: they used PCR to confirm RSV-LRTD? REALLY? I haven’t seen anyone mention this little item.Lower Respiratory Tract Disease (LRTD) was confirmed by PCR? That means that we don’t know what anyone had, since PCR cannot test for anything at all; it simply amplifies any part of a molecule to say you have the whole sickness. It’s like finding a molecule that has a piece of HCG, Human Chorionic Gonadotropin (the pregnancy test), and saying you are pregnant - when you are NOT!
Always remember: the PCR test is NOT like a pregnancy test that is never wrong!
For that reason, it doesn’t matter what the rest of their results showed, because the study design is flawed. We knew it was a stupid study, because they really have no way of knowing if this “cold symptom”, “mild cold” sickness is a coronavirus, respiratory syncytial virus, or other virus.
Of course, their data displays “efficacy” at preventing someone from getting sick with an RSV cold.
Patient Counseling.
This information is split into two sections: the first visits the information site, and the second leads to the professional link that Moderna uses to list updated shot info.
“Advise the vaccine recipient or caregiver to read the FDA-approved patient labeling (INFORMATION FOR RECIPIENTS AND CAREGIVERS).”
A. Here is the archived site; note it is for SPIKEVAX, Moderna’s Covid jab that causes myocarditis (i.e., Moderna didn’t have to write a new manual for MRESVIA; instead, they refer to the old SPIKEVAX Moderna Covid jab)!!
The pdf link at the bottom of the above page is HERE: https://dailymed.nlm.nih.gov/dailymed/
Source: https://static.modernatx.com/pm/6cef78f8-8dad-4fc9-83d5-d2fbb7cff867/36130c97-6fb0-4bea-9f2e-fb5be7a90729/36130c97-6fb0-4bea-9f2e-fb5be7a90729_viewable_rendition__v.pdf
B. For updates to mRESVIA®, I first visited here: https://www.modernatx.com/en-US/products/mresvia:
I clicked on the section for Healthcare Professionals, HERE:
Interesting that they cite GlaxoSmithKline and Pfizer’s RSV jabs in their references.
Under their disclosures, they list a link for those in Colorado, that shows the PRICE of mRESVIA. 10 vials in one package costs $290/syringe = $2,900.
https://modernadirect.com/wac-disclosure
You should know that ALL pseudouridinated, synthetic mRNA products, including Moderna’s mRESVIA®, have the same precautions.
Dr. Peter McCullough cited these studies that back up the above statement:
Auto-immunity. Boros et al showed that foreign RSV proteins and frameshifting peptides adversely effect the immune system.
Genomic integration. as shown by Aldén et al showed that it alters liver genes, and no one has refuted it.
Myocarditis. Krauson et al showed they target the heart.
Oncogenicity. Seneff et al showed that they cause cancer or allow old cancers to regrow.
Now I think that’s enough of a reason not to trust that anyone anywhere needs this jab.
References
1. The CED Website on RSV
https://www.cdc.gov/vaccines/hcp/vis/vis-statements/rsv.html
Why get vaccinated?
RSV vaccine can prevent lower respiratory tract disease caused by respiratory syncytial virus (RSV). RSV is a common respiratory virus that usually causes mild, cold-like symptoms.
RSV can cause illness in people of all ages but may be especially serious for infants and older adults.
Infants up to 12 months of age (especially those 6 months and younger) and children who were born prematurely, or who have chronic lung or heart disease or a weakened immune system, are at increased risk of severe RSV disease.
Adults at highest risk for severe RSV disease include older adults, adults with chronic medical conditions such as heart or lung disease, weakened immune systems, or certain other underlying medical conditions, or who live in nursing homes or long-term care facilities.
RSV spreads through direct contact with the virus, such as droplets from another person’s cough or sneeze contacting your eyes, nose, or mouth. It can also be spread by touching a surface that has the virus on it, like a doorknob, and then touching your face before washing your hands.
Symptoms of RSV infection may include runny nose, decrease in appetite, coughing, sneezing, fever, or wheezing. In very young infants, symptoms of RSV may also include irritability (fussiness), decreased activity, or apnea (pauses in breathing for more than 10 seconds).
Most people recover in a week or two, but RSV can be serious, resulting in shortness of breath and low oxygen levels. RSV can cause bronchiolitis (inflammation of the small airways in the lung) and pneumonia (infection of the lungs). RSV can sometimes lead to worsening of other medical conditions such as asthma, chronic obstructive pulmonary disease (a chronic disease of the lungs that makes it hard to breathe), or congestive heart failure (when the heart can’t pump enough blood and oxygen throughout the body).
Older adults and infants who get very sick from RSV may need to be hospitalized. Some may even die.
RSV vaccine
CDC recommends adults 60 years of age and older have the option to receive a single dose of RSV vaccine, based on discussions between the patient and their health care provider.
There are two options for protection of infants against RSV: maternal vaccine for the pregnant person and preventive antibodies given to the baby. Only one of these options is needed for most babies to be protected. CDC recommends a single dose of RSV vaccine for pregnant people from week 32 through week 36 of pregnancyfor the prevention of RSV disease in infants under 6 months of age. This vaccine is recommended to be given from September through January for most of the United States. However, in some locations (the territories, Hawaii, Alaska, and parts of Florida), the timing of vaccination may vary as RSV circulating in these locations differs from the timing of the RSV season in the rest of the U.S.
RSV vaccine may be given at the same time as other vaccines.
Talk with your health care provider
Tell your vaccination provider if the person getting the vaccine:
Has had an allergic reaction after a previous dose of RSV vaccine, or has any severe, life-threatening allergies
In some cases, your health care provider may decide to postpone RSV vaccination until a future visit.
People with minor illnesses, such as a cold, may be vaccinated. People who are moderately or severely ill should usually wait until they recover before getting RSV vaccine.
Your health care provider can give you more information.
Risks of a vaccine reaction
Pain, redness, and swelling where the shot is given, fatigue (feeling tired), fever, headache, nausea, diarrhea, and muscle or joint pain can happen after RSV vaccination.
Serious neurologic conditions, including Guillain-Barré syndrome (GBS), have been reported after RSV vaccination in clinical trials of older adults. It is unclear whether the vaccine caused these events.
Preterm birth and high blood pressure during pregnancy, including pre-eclampsia, have been reported among pregnant people who received RSV vaccine during clinical trials. It is unclear whether these events were caused by the vaccine.
People sometimes faint after medical procedures, including vaccination. Tell your provider if you feel dizzy or have vision changes or ringing in the ears.
As with any medicine, there is a very remote chance of a vaccine causing a severe allergic reaction, other serious injury, or death.
What if there is a serious problem?
An allergic reaction could occur after the vaccinated person leaves the clinic. If you see signs of a severe allergic reaction (hives, swelling of the face and throat, difficulty breathing, a fast heartbeat, dizziness, or weakness), call 9-1-1and get the person to the nearest hospital.
For other signs that concern you, call your health care provider.
Adverse reactions should be reported to the Vaccine Adverse Event Reporting System (VAERS). Your health care provider will usually file this report, or you can do it yourself. Visit the VAERS website or call 1-800-822-7967. VAERS is only for reporting reactions, and VAERS staff members do not give medical advice.
How can I learn more?
Ask your health care provider.
Call your local or state health department.
Visit the website of the Food and Drug Administration (FDA) for vaccine package inserts and additional information.
Contact the Centers for Disease Control and Prevention (CDC):
Call 1-800-232-4636 (1-800-CDC-INFO) or
Visit CDC’s vaccine website
Many vaccine information statements are available in Spanish and other languages. See www.immunize.org/vis
Hojas de información sobre vacunas están disponibles en español y en muchos otros idiomas. Visite www.immunize.org/vis
Vaccine Information Statement
RSV Vaccine
(10/19/2023)Department of Health and Human Services
Centers for Disease Control and PreventionOffice Use Only
Source: https://www.cdc.gov/vaccines/hcp/vis/vis-statements/rsv.html
2. Package Insert, MRESVIA
Source: https://static.modernatx.com/pm/6cef78f8-8dad-4fc9-83d5-d2fbb7cff867/36130c97-6fb0-4bea-9f2e-fb5be7a90729/36130c97-6fb0-4bea-9f2e-fb5be7a90729_viewable_rendition__v.pdf
Source: https://static.modernatx.com/pm/6cef78f8-8dad-4fc9-83d5-d2fbb7cff867/36130c97-6fb0-4bea-9f2e-fb5be7a90729/36130c97-6fb0-4bea-9f2e-fb5be7a90729_viewable_rendition__v.pdf
Thank you for supporting my writings, even if you were previously paid and now I switched to free subscriptions. I want you to know that no one so far has unsubscribed because of this change. And I so much appreciate your dedication to my writings. For those of you who don’t know me quite well yet, please know that if I am “late” in writing an article, you can bet that it’s because I am researching and writing:)! Thank you for letting me take my weekends off - if I am not occupied with family and/or if something crazy comes up, I do write then. Being as we are newly homesteading, I usually have a bit of dirt under my fingernails on the weekends! All Blessings!








































Ta1 - Never heard of it! THIS ⬆️ is fascinating! Please write a longer article on it, and include pictures of its molecular structure.
Quite interesting. And the relationship to IVIG?
Remember they put out monoclonal antibodies (MAb) for a time with Covid; Florida was using them a lot, then Biden split it up to give other states a supply.
I stopped prescribing it when I learned it came from aborted tissue from live births. And you never hear of people who suffer any sequelae from MAb. Why not, if it's a blood product containing such screaming cell lines as the HEK293.
One of my very good friends had the RSV jab and felt like she’d been poisoned!!! She called her daughter who’s a nurse and exclaimed she felt very strange and oddly sick while walking around the store as she’d gotten it at a local pharmacy. It took her several weeks to BEGIN to feel somewhat better. She’s now awake, but it took THIS to get her there!!